Compound and method for the prevention and/or the treatment of allergy
An allergy, compound technology, applied in the direction of fusion polypeptides, animal feed, cosmetic preparations, etc., can solve problems such as loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] A peptide of 31 amino acids consisting of 15 amino acids representative of the T-cell epitope of tetanus toxoid (amino acids 830-844 of the heavy chain) and 14 amino acids of the B-cell epitope comprising Der p II was obtained by synthesis, wherein The two epitopes are distinguished by a stretch of two glycine residues. The sequence is SEQ ID NO 1: QYIKANSKFIGITELGGHEIKKVLVPGCHGS.
[0103] Peptide Characterization
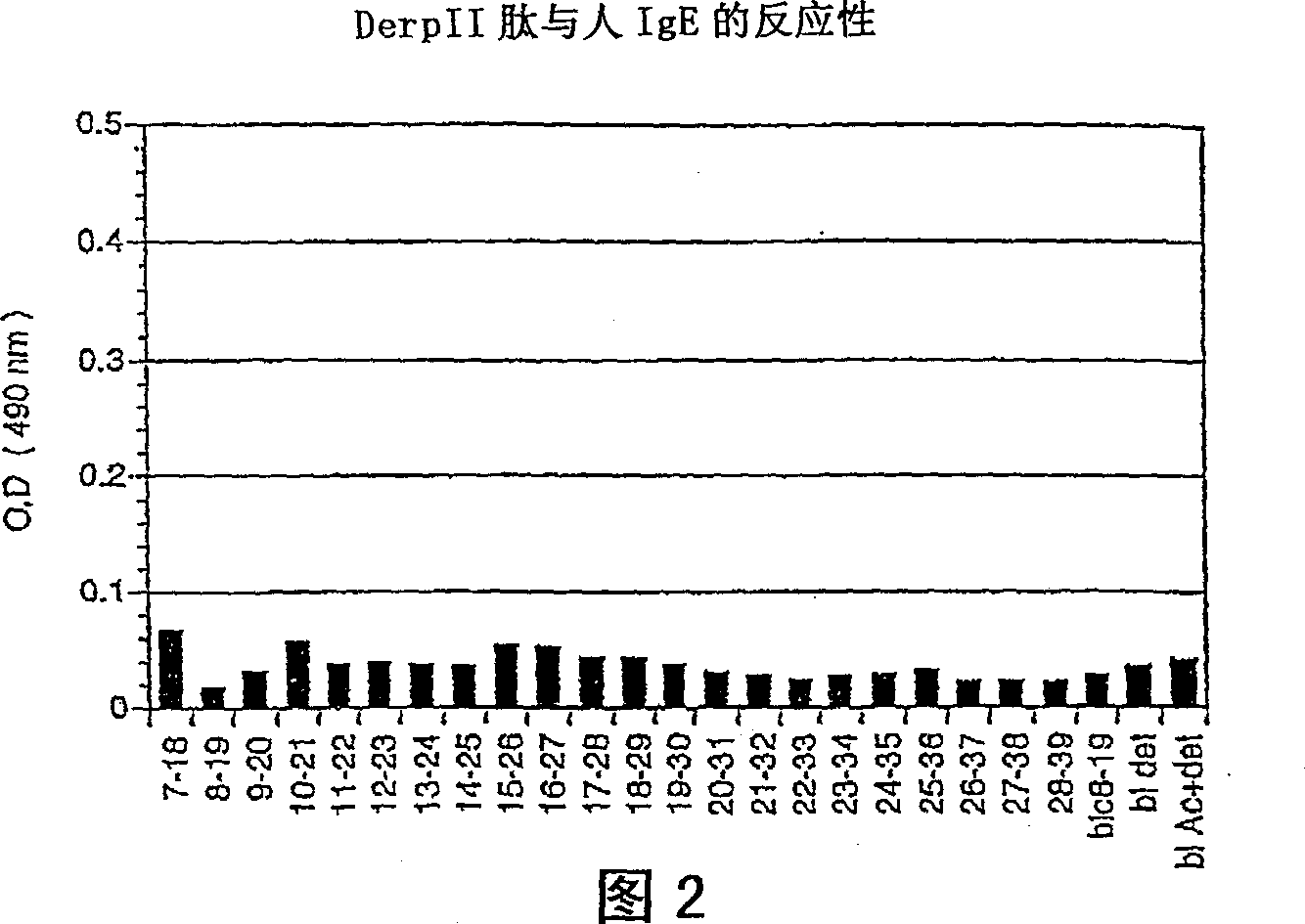
[0104] 1. IgE antibodies do not recognize this B cell epitope
[0105] IgE antibodies produced by individuals sensitive to the native protein do not recognize the peptide. This was demonstrated by immunoassays performed as follows. The peptides were immobilized on polystyrene microtiter plates and added in parallel to serum samples of atopic individuals sensitive to Der p II; binding of specific IgE antibodies was detected by addition of isotype-specific reagents.
[0106]Thus, a peptide (SEQ ID NO. 2) corresponding to the sequence HEIKKVLVPGCHGS of ami...
Embodiment 2
[0117] The compounds of the invention are prepared by recombinant cDNA techniques that generate polypeptides consisting of a series of repeating units comprising T and B cell epitope peptides. A polypeptide consisting of repeated T-cell epitopes derived from TT (amino acids 830-844 of the heavy chain) and six repeated B-cell epitopes derived from Der p II was prepared by DNA technology. A sequence of two amino acid residues is inserted between each epitope. The sequence is: D-(QYIKANSKFIGITELX)2-(CHGSEPCIIHRGKPFX)5-CHGSEPCIIHRGKPFSR,
[0118] where X is GG or SS.
[0119] Such polypeptides are obtained as follows. The nucleotide sequence corresponding to the TT epitope of QYIKANSKFIGITEL (SEQ ID NO. 13) and the nucleotide sequence of Der p II epitope 21-35 corresponding to CHGSEPCIIHRGKPF (SEQ ID NO. 14) were deduced. Theoretical assembly was performed from nucleotides corresponding to the sequence TT epitope-GG-TT epitope (T subunit) on the one hand and two copies of the D...
Embodiment 3
[0139] Nucleotide sequences encoding the compounds of the present invention can be used in direct genetic immunization. DNA-based vaccines can be administered by different routes (i.e., intramuscular, intradermal, subcutaneous, orally) using "naked" DNA, encapsulated DNA, or DNA in the form of micro- or nano-sized particles such as chitosan (K. Roy et al., Nature Medicine 1999; 5:387-391).
[0140] For direct intramuscular immunization, a nucleotide construct prepared according to Example 2 but comprising a DNA sequence encoding one T-cell epitope derived from TT and two B-cell epitopes derived from Der p II was used, Each epitope is separated by the sequence GGAGGT or GGCGGT encoding 2 glycine residues. The nucleotide sequence is flanked by a sequence comprising an EcoRI restriction enzyme site and a KOZAK sequence (i.e. GAATTCCCACCATGG (SEQ ID NO. 16)) at the 5' end and flanked by a stop codon and A NotI restriction site (ie TAGGCGGCCGC (SEQ ID NO. 17)), and inserted into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com