Novel type sugar of C12 high carbon, its derivative, preparation method and application
A sugar derivative, C12 technology, applied in the field of sugar compounds and their preparation, can solve the problems of less high aldose, no synthetic high carbon ketose, etc., and achieves mild conditions, good market prospects, and high reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
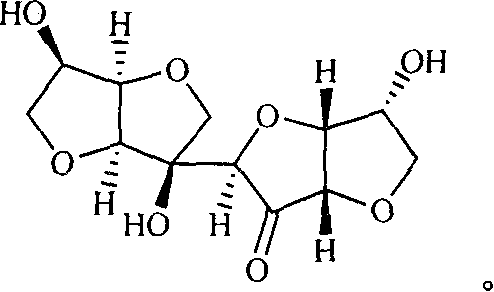
[0032] Example 1 Preparation C 12 high carbon ketose
[0033] 1,4:3,6-Fruitose (1.44g, 10mmol) was dissolved in methanol (10mL), a catalytic amount of sodium methoxide was added, and the mixture was refluxed for 6 hours. After the reaction, the reaction solution was concentrated, crystallized from absolute ethanol, and C was obtained. 12 High carbon ketose 1.15g, yield 80%. C 12 The data of the high-carbon ketose experiment are as follows.
[0034] C 12 h 16 o 8 , mp 190-191°C, [α] D 20 =+173.4(c 1.10, CH 3 OH); υ=3381,1771,1401,1128,1066,880,747cm -1 ; 1 HNMR (400MHz, DMSO-d 6 ): δ4.87(dd, J=4.8, 6.8Hz, 1H), 4.39-4.41(m, 2H), 4.26(d, J=6.8Hz, 1H), 4.10-4.15(m, 2H), 3.92( s, 1H), 3.95(d, J=8.8Hz, 1H), 3.82(dd, J=4.8, 8.8Hz, 1H), 3.79(m, 1H), 3.54(dd, J=4.8, 8.8Hz, 1H ), 3.42(d, J=8.8Hz, 1H), 3.38(m, 1H); 13 CNMR (100MHz, DMSO-d 6): δ212.8, 83.2, 81.4, 81.0, 80.3, 79.7, 78.4, 72.5, 72.3, 72.2, 71.8, 71.3.
Embodiment 2
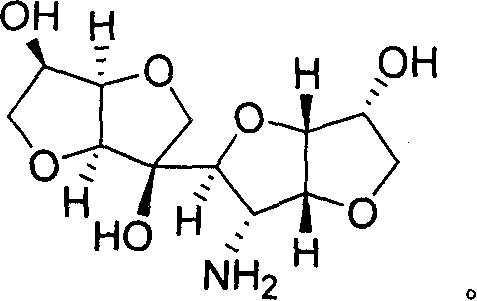
[0035] Example 2 Preparation of Derivatives When X=O Shown in General Formula 1
[0036] The above prepared C 12 High carbon sugar (1.44g, 5mmol) was dissolved in ethanol (20mL), 0.5mL of nitromethane and 20mg of potassium fluoride were added, and reacted at 80°C for 5 hours. After the reaction was completed, the reaction solution was concentrated and recrystallized from methanol to obtain 1.66 g of the derivative represented by general formula 1 when X=O, with a yield of 95%. The experimental data are as follows.
[0037] C 13 h 19 NO 10 , mp 160-162°C, [α] D 20 =+92.9 (c=1.06, CH 3 OH), υ=3405, 2942, 2849, 1554, 1416, 1123, 1075, 859, 702cm -1 ; 1 HNMR (400MHz, D 2 O): δ4.95(d, J=13.6Hz, 1H), 4.85(d, J=13.6Hz, 1H), 4.60(t, J=4.0Hz, 1H), 4.59(d, J=5.4Hz, 1H), 4.48(d, J=4.0Hz, 1H), 4.31(m, 1H), 4.26(t, J=5.4Hz, 1H), 4.17(m, 1H), 3.91(s, 2H), 3.89( dd, J=6.4, 8.8Hz, 1H), 3.86(dd, J=6.8, 8.8Hz, 1H), 3.78(s, 1H), 3.50(t, J=8.8Hz, 1H), 3.45(t, J = 8.8Hz, 1H); 13 CNMR...
Embodiment 3
[0038] Example 3 Preparation of Derivatives When X=H Shown in General Formula 1
[0039] The product obtained in Example 2 (1.05 g, 3 mmol) was dissolved in methanol (120 mL), a catalyst 10% Pd / C (105 mg) was added, and hydrogenation was performed at 40° C. for 8 hours. After the reaction was completed, the reaction liquid was concentrated and crystallized from acetonitrile to obtain 852 mg of the derivative represented by general formula 1 when X=H, with a yield of 89%. The experimental data are as follows.
[0040] C 13 h 21 NO 8 , mp 76-78°C, [α] D 20 =+86.5 (c=0.32, CH 3 OH), υ=3367, 2946, 2872, 1131, 1082, 1042, 866cm -1 ; 1 HNMR (400MHz, D 2 O): δ4.66(d, J=4.4Hz, 1H), 4.65(t, J=4.4Hz, 1H), 4.32(m, 1H), 4.31(t, J=4.4Hz, 1H), 4.28( d, J=4.4Hz, 1H), 4.24(m, 1H), 3.95(s, 2H), 3.94(m, 1H), 3.87(s, 1H), 3.85(dd, J=6.4, 9.2Hz, 1H ), 3.58(t, J=8.4Hz, 1H), 3.52(dd, J=7.2, 9.2Hz, 1H), 3.28(s, 2H); 13 CNMR (100MHz, D 2 O): δ88.1, 85.9, 85.3, 80.9, 80.8, 80.4, 79.7, 74....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com