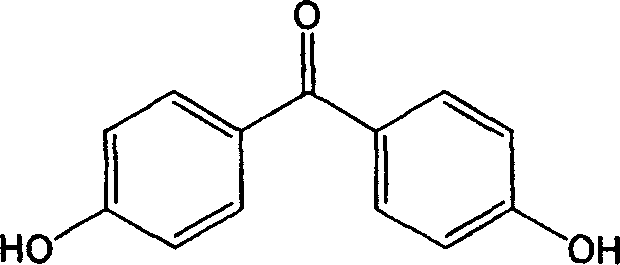
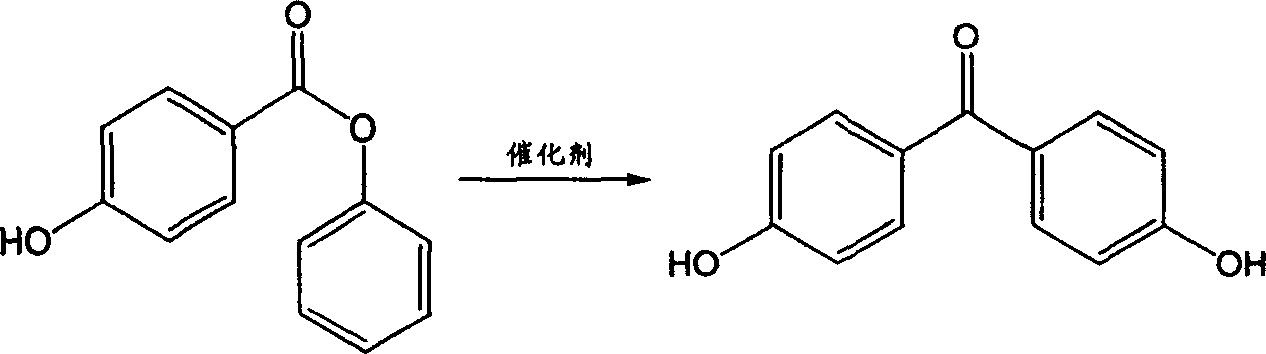
Method for preparing 4,4'-dihydroxy benzophenone
A technology of dihydroxybenzophenone and phenyl hydroxybenzoate, which is applied in 4 fields, can solve the problems of limited catalyst types, serious environmental pollution, and difficult recovery of waste phosphoric acid, etc., to reduce production costs, simplify processes, and achieve high yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) p-acetoxybenzoic acid
[0017] Add p-hydroxybenzoic acid and acetic anhydride into a 250ml three-necked flask. After stirring, add a catalytic amount of concentrated sulfuric acid. Heat at 65°C to react for 2h. Discharge the material and pour it into ice water. Stir to separate the solid and filter. Recrystallize from ethanol to obtain a white solid. P-acetoxybenzoic acid, the yield is 93%, mp192~194.
[0018] (2) p-acetoxybenzoyl chloride
[0019] Add 28g (0.16mol) of p-acetoxybenzoic acid, 40ml (0.55mol) of thionyl chloride and pyridine (0.06~0.1ml) in a three-necked flask equipped with a stirring and reflux condenser, and raise the temperature to 70~75℃ The reaction was stirred for 3h until no gas escaped. After cooling to room temperature, the unreacted thionyl chloride was distilled off, and the residue was distilled under reduced pressure to collect the 140-144°C / 10.67kPa fraction to obtain 29 g (0.15 mol) of light yellow oily liquid p-acetoxybenzoyl chloride.
[...
Embodiment 2
[0027] The preparation methods of p-acetoxybenzoic acid, p-acetoxybenzoyl chloride, and phenyl p-hydroxybenzoate are the same as in Example 1.
[0028] Chlorobenzene was used as the solvent, and the reaction was refluxed for 10 hours to prepare phenyl p-acetoxybenzoate with a yield of 96%.
[0029] The ratio of phenyl p-hydroxybenzoate: trifluoromethanesulfonic acid is 1:0.2 (molar ratio), petroleum ether is the solvent, and the reaction is heated and stirred at reflux for 10 hours. Wash with 10% sodium bicarbonate solution and water, and recrystallize from methanol-water (1:3, volume ratio) to obtain the target compound 4,4'-p-dihydroxybenzophenone. The yield was 98%. mp219~220℃.
Embodiment 3
[0031] The preparation methods of p-acetoxybenzoic acid, p-acetoxybenzoyl chloride, and phenyl p-hydroxybenzoate are the same as in Example 1.
[0032] Chlorobenzene was used as the solvent, and the reaction was refluxed for 10 hours to prepare phenyl p-acetoxybenzoate with a yield of 96%.
[0033] Phenyl p-hydroxybenzoate: chromium trifluoromethanesulfonate is 1:0.15 (molar ratio), cyclohexane is the solvent, and the reaction is heated and stirred at reflux for 8 hours. Wash with 10% sodium bicarbonate solution and water, and recrystallize from methanol-water (1:3, volume ratio) to obtain the target compound 4,4'-p-dihydroxybenzophenone. The yield was 99%. mp219~220℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com