Method for preparing 1-ferrocenyl-3-aryl-3-diacetyl methylene-acetone
A technology of diacetylmethine and ferrocene, applied in the field of chemical synthesis, can solve the problems of large amount of strong base, low yield, and inability to recycle and use, and achieves overcoming the large amount of consumption, low cost of raw materials, and ease of use. the effect obtained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11- 2
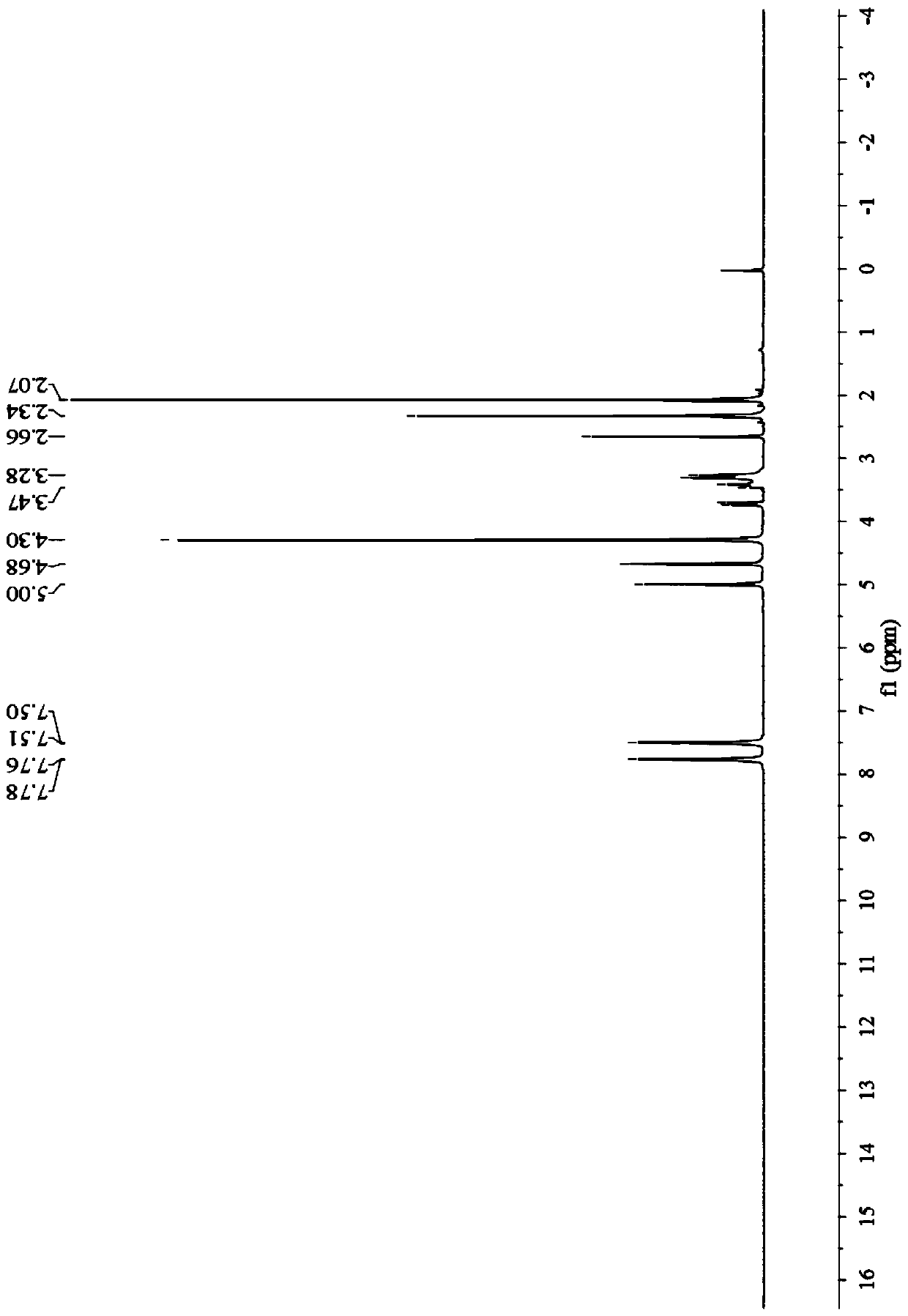
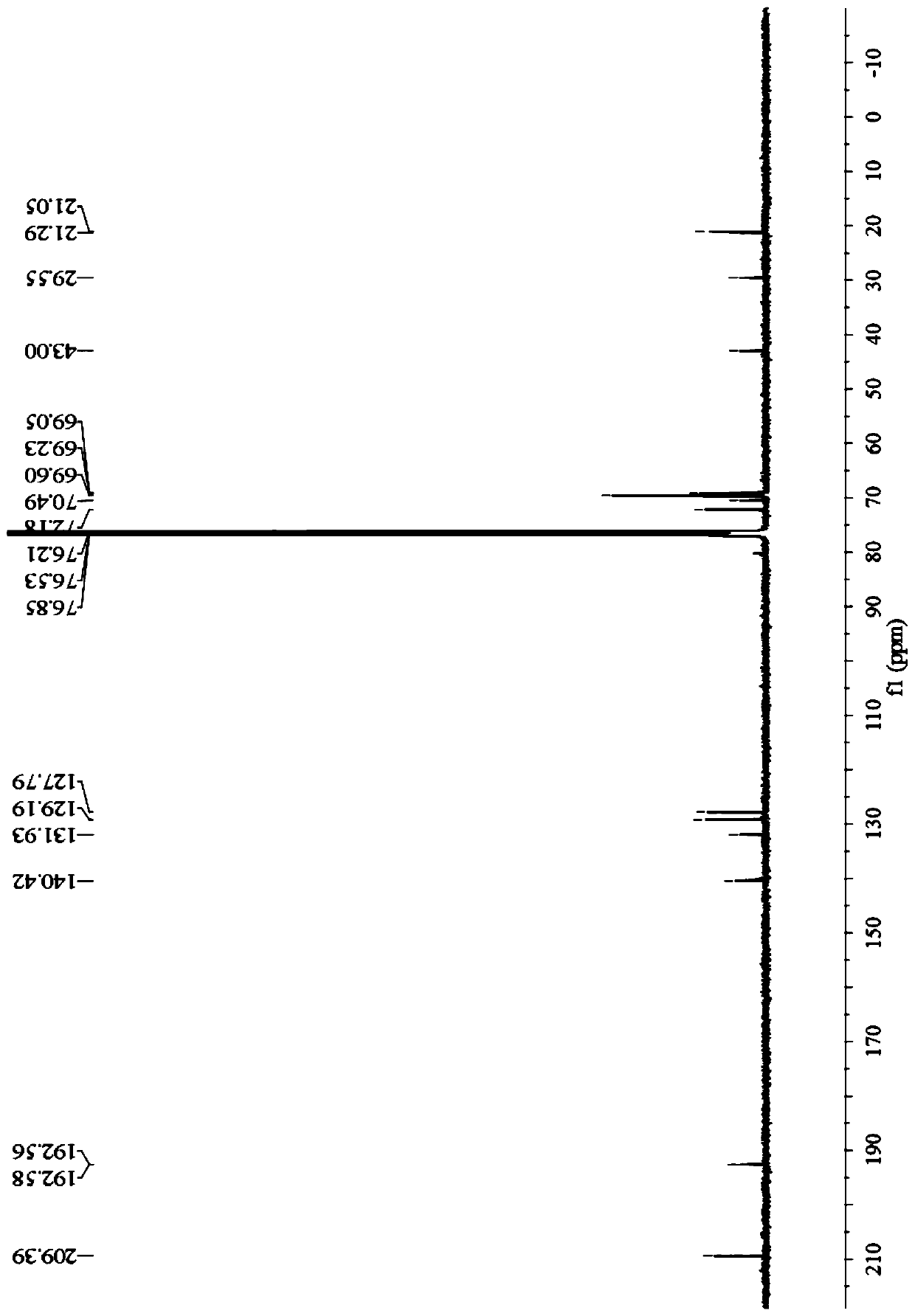
[0043] Preparation of Example 11-ferrocenyl-3-phenyl-3-diacetylmethine-acetone:
[0044]
[0045] In the first step, add 1mol choline chloride and 2mol urea to the reaction vessel, stir at 80°C until completely dissolved to obtain a deep eutectic solvent;
[0046] In the second step, after cooling the reaction system to room temperature, add 0.01mol 1-ferrocenyl-3-aryl-propenone and 0.012mol 2,4-pentanedione, slowly heat up, reflux reaction, TLC monitoring until the end of the reaction (25min);
[0047] In the third step, the reaction solution was cooled to room temperature, and a solid was precipitated, which was suction filtered, and the filter cake was washed with a small amount of water to obtain 1-ferrocenyl-3-phenyl-3-diacetylmethine-acetone. The yield was 83.4%, m.p.: 106-108°C; the filtrate was recovered to obtain a deep eutectic solvent. The yield of the deep eutectic solvent was 83.2% for the first time, 83% for the second time, 82.8% for the third time, and 82....
Embodiment 21- 2
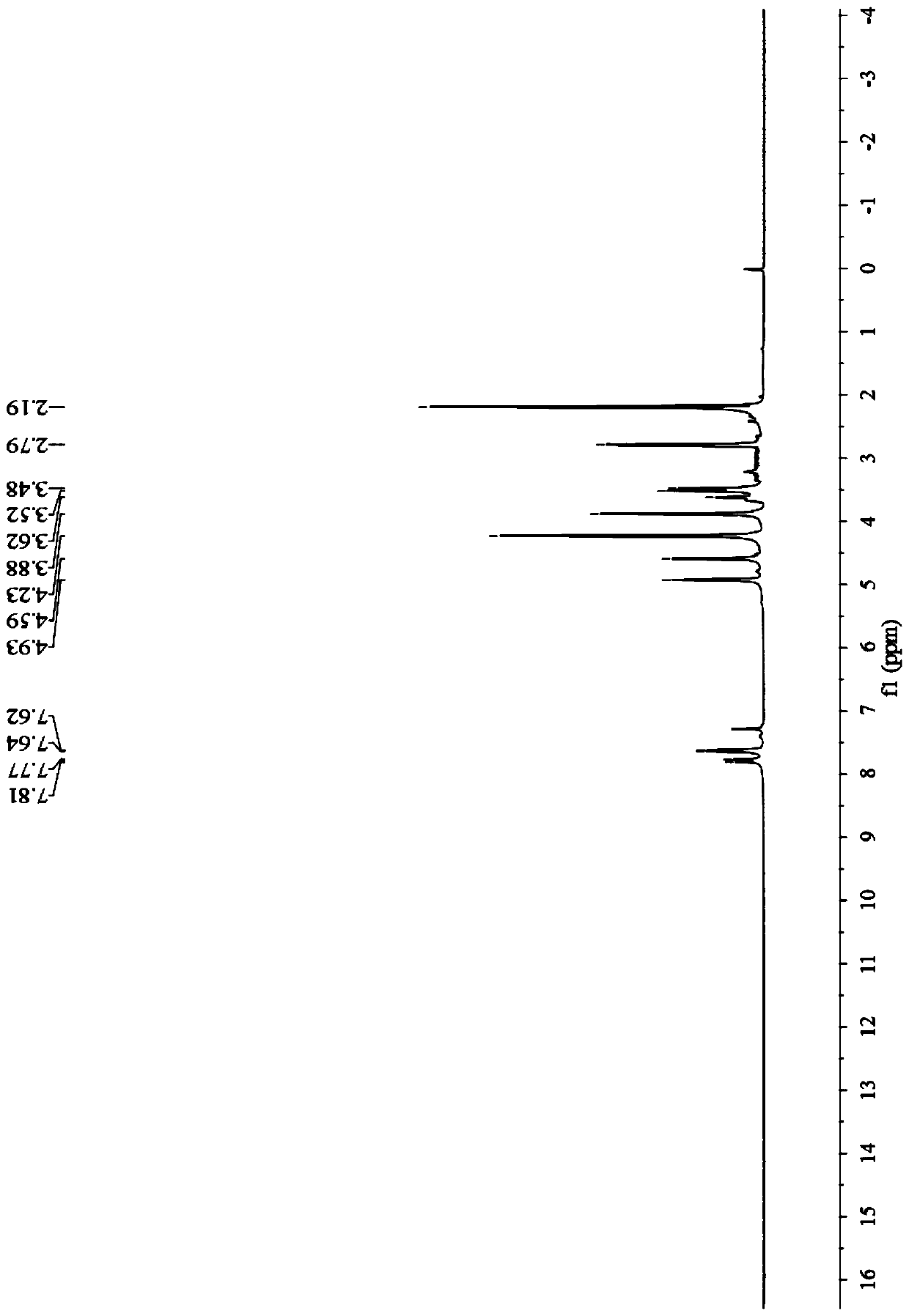
[0052] Example 21- Preparation of ferrocenyl-3-(p-fluorophenyl)-3-diacetylmethine-acetone:
[0053]
[0054] In the first step, add 1mol choline chloride and 2mol urea to the reaction vessel, stir at 80°C until completely dissolved to obtain a deep eutectic solvent;
[0055] In the second step, after cooling the reaction system to room temperature, add 0.01mol 1-ferrocenyl-3-(p-fluorophenyl)-propenone and 0.012mol 2,4-pentanedione, slowly heat up, reflux reaction, TLC Monitor until the end of the reaction (25min);
[0056] In the third step, the reaction liquid is cooled to room temperature, and a solid is precipitated, filtered with suction, and the filter cake is washed with a small amount of water to obtain 1-ferrocenyl-3-(p-fluorophenyl)-3-diacetylmethine-acetone . The yield is 91%, m.p.: 151-153°C; the filtrate is recovered to obtain a deep eutectic solvent. The yield of the deep eutectic solvent was 89.9% for the first time, 89.5% for the second time, 89.1% for the t...
Embodiment 31- 2
[0061] Example 31- Preparation of ferrocenyl-3-(p-chlorophenyl)-3-diacetylmethine-acetone:
[0062]
[0063] In the first step, add 1mol choline chloride and 2mol urea to the reaction vessel, stir at 80°C until completely dissolved to obtain a deep eutectic solvent;
[0064] In the second step, after cooling the reaction system to room temperature, add 0.01mol 1-ferrocenyl-3-(p-chlorophenyl)-propenone and 0.012mol 2,4-pentanedione, slowly heat up, reflux reaction, TLC Monitor until the end of the reaction (30min);
[0065] In the third step, the reaction solution was cooled to room temperature, and a solid was precipitated, filtered with suction, and the filter cake was washed with a small amount of water to obtain 1-ferrocenyl-3-(p-chlorophenyl)-3-diacetylmethine-acetone . The yield was 93.2%, m.p.: 147-149°C; the filtrate was recovered to obtain a deep eutectic solvent. The yield of the deep eutectic solvent was 93% for the first time, 92.8% for the second time, 92.5% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com