Compound paracetamol and chlorphenamine maleate slow releasing tablet and its preparation
A technology for compound paracetamol sustained-release tablets and chlorpheniramine acid, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of short half-life, inconvenience in taking medicine, and affecting the therapeutic effect. and other problems, to achieve the effect of reducing toxic side effects, reducing the number of times of medication, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
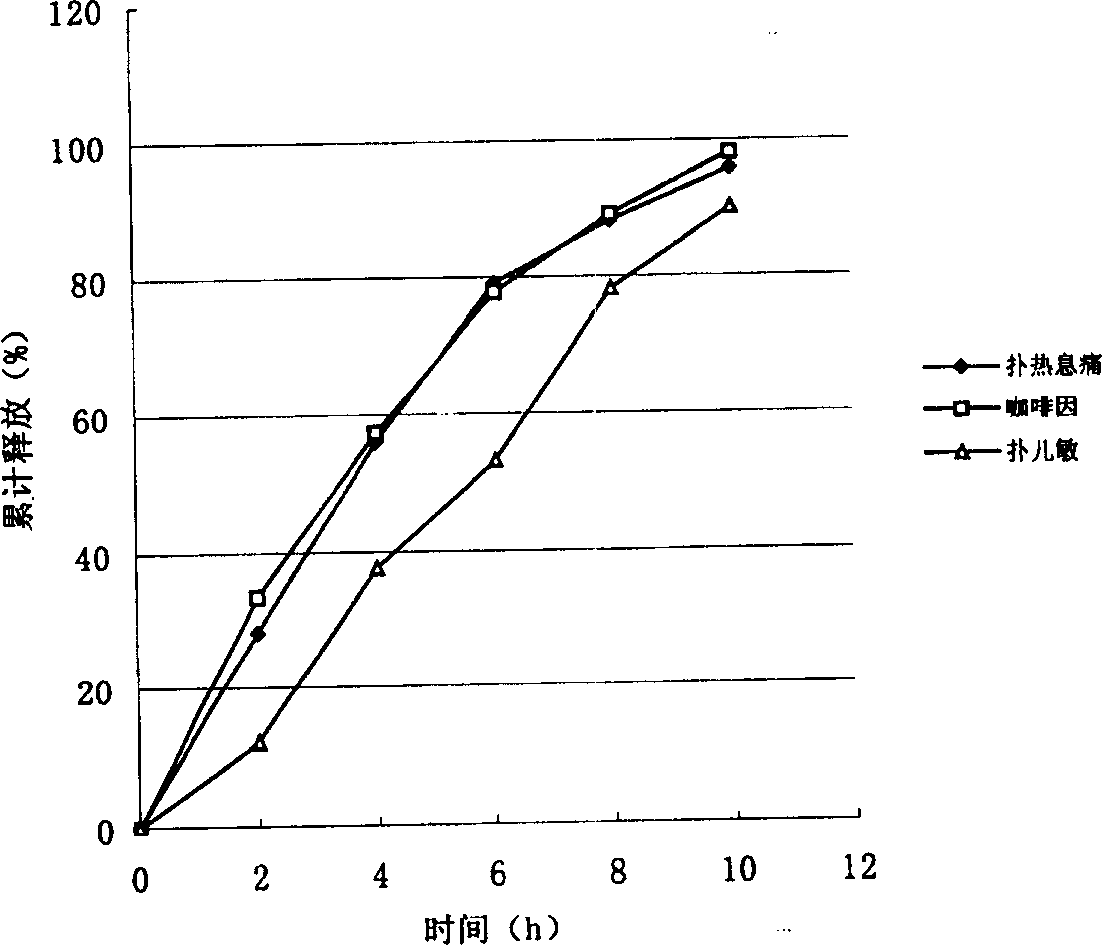
Image
Examples
Embodiment 1
[0027] Tablet prescription:
[0028] Acetaminophen 375g
[0029] Chlorpheniramine Maleate 4.5g
[0030] Caffeine 22.5g
[0031] Artificial Bezoar 15g
[0032] Carbomer 934P 159g
[0033] Povidone K30 18g
[0036] Preparation process (1) method: paracetamol, chlorpheniramine maleate, caffeine, and artificial bezoar are pulverized and sieved for each use. Povidone K30, magnesium stearate, and talcum powder were sieved separately for subsequent use. Put chlorpheniramine maleate, acetaminophen, caffeine, artificial bezoar, carbomer 934P, povidone K30 in the fluidized bed, mix well, and use absolute ethanol as the wetting agent in the spray In the state, make granules in one step, after drying in a fluidized bed, add magnesium stearate, talcum powder and mix well, then press into tablets, wrap with moisture-proof film, increase in weight by 3%, after drying, discharge the material and dry in a drying room at 40°C for 8 ...
Embodiment 2
[0042] Tablet core prescription
[0043] Acetaminophen 3750g
[0044] Chlorpheniramine Maleate 45g
[0045] Caffeine 225g
[0046] Artificial Bezoar 150g
[0047] Carbomer 934P 159g
[0048] Povidone K30 18g
[0050] Talc powder 3g
[0051] Preparation method one: crush and sieve acetaminophen, chlorpheniramine maleate, caffeine, and artificial bezoar for later use. Povidone K30, magnesium stearate, and talcum powder were sieved separately for subsequent use. Put chlorpheniramine maleate, acetaminophen, caffeine, artificial bezoar, carbomer 934P, povidone K30 in the fluidized bed, mix well, and use absolute ethanol as the wetting agent in the spray In the state, make granules in one step, after drying in a fluidized bed, add magnesium stearate and talc powder, mix well, press into tablets, wrap with a moisture-proof film, increase the weight by 3%, discharge after 20 minutes after drying, and dry at 40°C Let it dry indoors for 8 hours. ...
Embodiment 3
[0054] Tablet core prescription
[0055] Acetaminophen 1125g
[0056] Chlorpheniramine Maleate 13.5g
[0057] Caffeine 67.5g
[0058] Artificial Bezoar 45g
[0059] Hypromellose 4KM 477g
[0060] Povidone K30 54g
[0062] Talc powder 9g
[0063]Preparation process (1): crush and sieve acetaminophen, chlorpheniramine maleate, caffeine, and artificial bezoar for later use. Povidone K30, magnesium stearate, and talcum powder were sieved separately for subsequent use. Place chlorpheniramine maleate, acetaminophen, caffeine, artificial bezoar, carbomer 934P, and povidone K30 in a fluidized bed, mix well, and mix with absolute ethanol: water (1:1 ) is a wetting agent in a spray state, and it is made into granules in one step. After drying in a fluidized bed, add magnesium stearate and talcum powder, mix well, press into tablets, wrap with a moisture-proof film, increase in weight by 3%, and discharge after drying Dry in a drying room at 40°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com