Lumbricus kinase of electrophoretical purity and its prepn and use
A lumbrokinase and electrophoresis technology, which is applied in the field of lumbrokinase and its preparation, can solve the problems of complex separation and purification process, difficulty in industrialized expansion of production and the like, and achieves the effects of high fibrinolytic activity, easy amplification and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
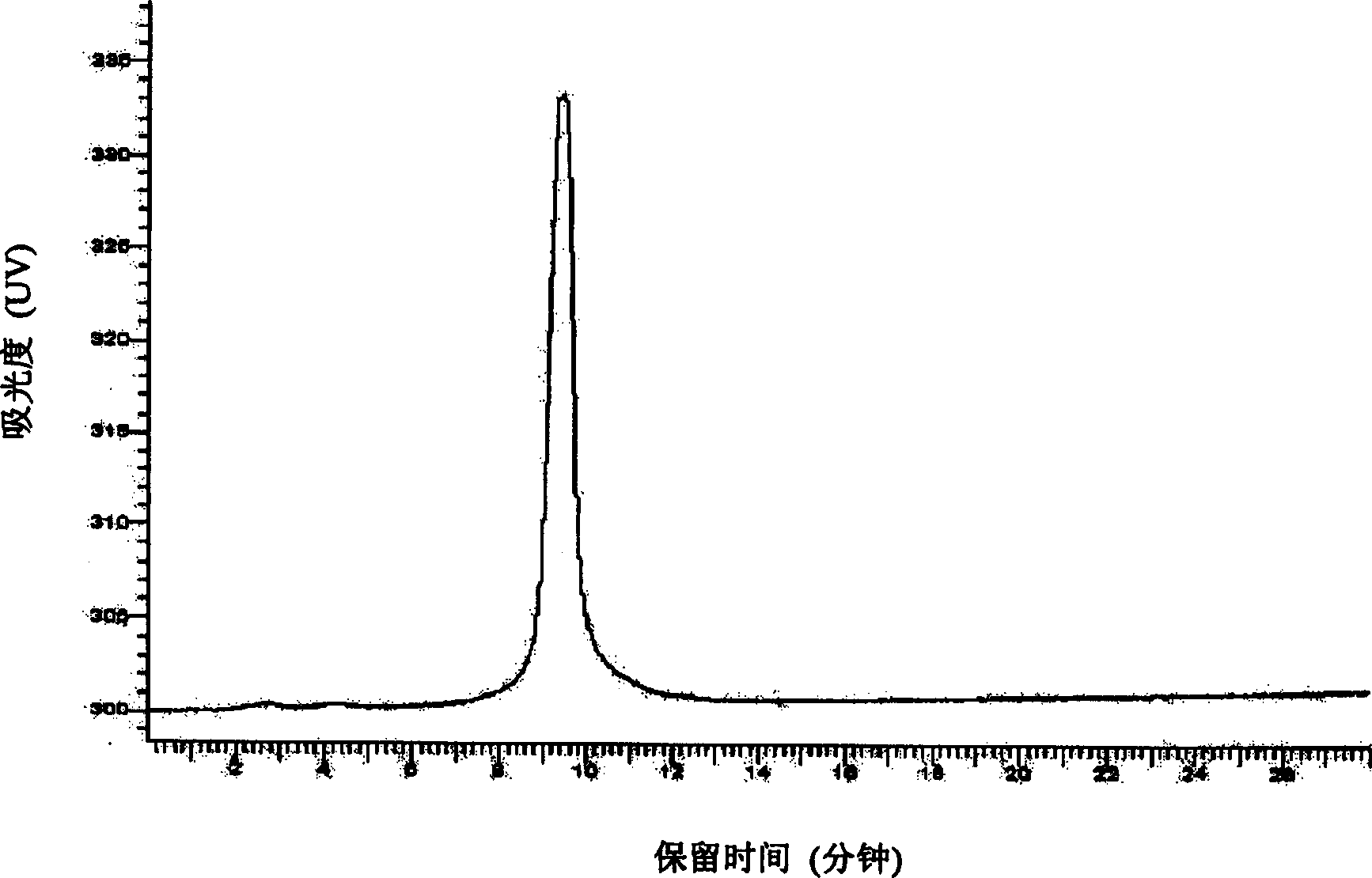
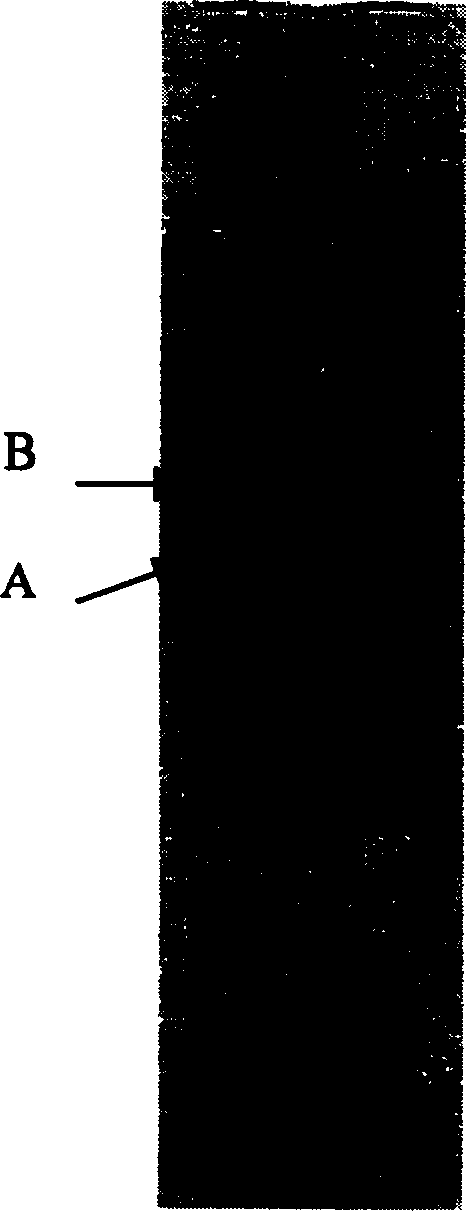
[0032] Take 5 grams of oral lumbrokinase raw material produced by Beijing Bio Pharmaceutical Co., Ltd., batch number 20010913-3, with a specific activity of 12090.8 units / mg, fully dissolve it with 0.02M phosphate buffer with pH 7.5 and set the volume to 100 mL, filter through a 0.45 μm microporous membrane to get the filtrate. A 20ml DEAESepharose Fast Flow anion exchange chromatography column was equilibrated with 0.02M phosphate buffer at pH 7.5, loaded with 20ml of sample, washed for one column volume, and eluted with a gradient of 0-0.5M NaCl phosphate buffer. The time is 30 minutes, and about 15 milliliters of protein peaks with fibrinolytic activity are collected. The samples obtained from 5 times DEAESepharose Fast Flow anion exchange chromatography were combined, desalted and concentrated with a 3K ultrafiltration cup, and replaced with 0.02M barbiturate monosodium chloride buffer solution with pH 7.8 to a volume of 10 ml. Use 20 milliliters of Q Sepharose HP anion c...
Embodiment 2
[0034] Get 10 grams of oral lumbrokinase crude drug produced by Beijing Baiao Pharmaceutical Co., Ltd., batch number 20010913-3, and the specific activity is 12090.8 units / mg, fully dissolved with 0.02M barbiturate-sodium chloride buffer solution of pH7.8 and The volume was adjusted to 100 ml, and the filtrate was obtained by filtration through a 0.45 μm microporous membrane. A 20ml Q Sepharose XL anion exchange chromatography column was equilibrated with 0.02M barbiturate-sodium chloride buffer at pH 7.8, loaded with 25ml of sample and washed for one column volume with 0-1M NaCl barbiturate-chloride buffer Gradient elution with sodium chloride buffer, the elution time is 50 minutes, and about 20 ml of protein peaks with fibrinolytic activity are collected. The samples obtained from the 4 times of Q Sepharose XL anion exchange chromatography column chromatography were combined, desalted and concentrated with a 3K ultrafiltration cup, and replaced with 0.02M Tris-HCl buffer sol...
Embodiment 3
[0036]Take 50 grams of oral lumbrokinase raw material produced by Beijing Baiao Pharmaceutical Co., Ltd., batch number 2002120, with a specific activity of 15507.6 units / mg, fully dissolve it with 0.02M phosphate buffer solution of pH 7.5 and make it to 1000 ml, 0.45 Filter the filtrate through a μm microporous membrane. A 300ml QSepharose XL anion exchange chromatography column was equilibrated with 0.02M phosphate buffer at pH 7.5, loaded with 500ml of sample, washed one column volume, and eluted with a gradient of 0-0.5M NaCl phosphate buffer, the elution time For 75 minutes, about 300 ml of protein peak with fibrinolytic activity was collected. The samples obtained from the two Q Sepharose XL anion-exchange chromatography column chromatography were combined and concentrated with a 5K ultrafiltration system, and replaced with 0.02M barbiturate-sodium chloride buffer solution with pH 7.8 to a volume of 200 ml. Use 300 milliliters of QSepharose HP anion-exchange chromatograp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com