Vitamin D precursors, preparation and intermediates thereof
A C1-C6, alkyl technology, applied in the field of synthesizing precursors of 19-nor-vitamin D analogs, can solve the problems of low molecular weight, volatile intermediates, difficult large-scale methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
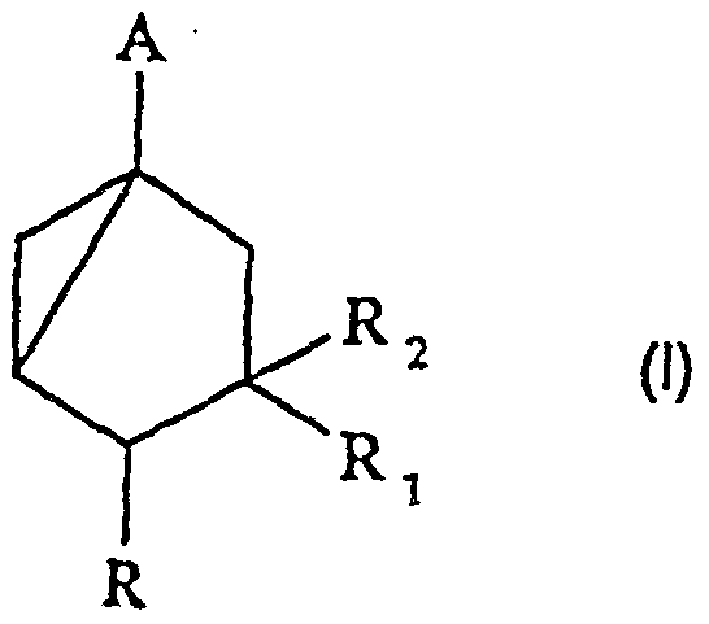
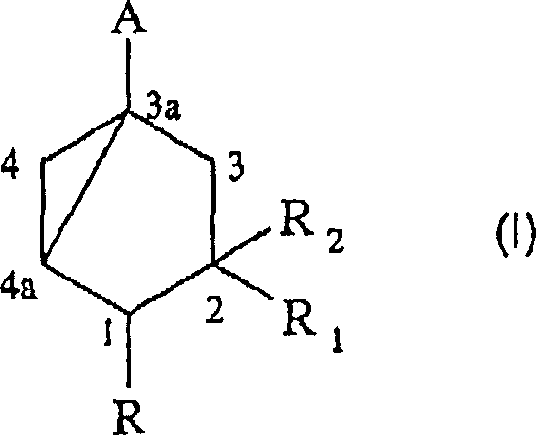
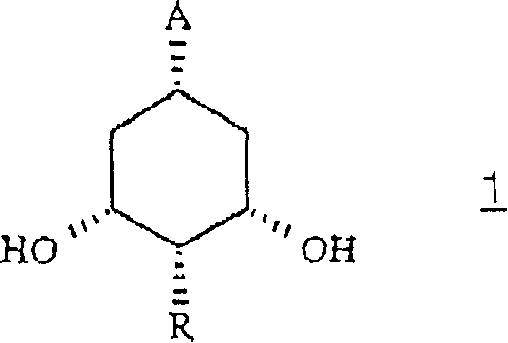
[0075] The preparation of formula 1 intermediate
[0076] a) cis, cis-3,5-dihydroxy-1-(methoxycarbonyl)cyclohexane:
[0077] 1. A(R=H, A-COOCH 3 ).
[0078] Hydrogenation of 3,5-dihydroxybenzene in MeOH under conditions similar to those described for the hydrogenation of 3,5-dihydroxybenzoic acid by Peng Wang and Julian Adams in J.Am.Chem.Soc. 1994, 116, 3296-3305 Methyl formate.
[0079] Methyl 3,5-dihydroxybenzoate (57.6 g, 0.629 mol, 97%) in MeOH (400 mL) containing 0.1% AcOH, 5% Rh / Al 2 Oh 3 (5.76 g) into the autoclave (1 L). Hydrogen (130 atm to 40 atm) was passed into the autoclave twice. The hydrogen pressure reaches 130 atmospheres and the temperature rises to 80-85°C. As the temperature rises, the hydrogen pressure drops. When the pressure drops to 90 atmospheres, the hydrogen pressure will return to 130 atmospheres. The hydrogenation is carried out at 80-85° C. and 130 atmospheres for 12 hours, then the temperature is raised to 150° C. and the corresponding...
Embodiment 1
[0134] Example 1: (2S, 3aS, 4aS)-2-tert-butyldimethylsilyloxy-3a-methoxycarboxy-bicyclo[3.1.0]hexane: I.b.1 (A=COOCH 3 , R=H, P=TBDMS).
[0135] a) Methyl (1R,3S,5R)-3-acetoxy-5-tosyloxy-cyclohexanecarboxylate: 2.4.a (R=H, L=OTos, A=COOCH 3 ).
[0136] p-Toluenesulfonyl chloride (13.3 g, 7.0 mmol) was added to (1S, 3S, 5R)-3-acetyl Oxygen-5-hydroxy-cyclohexanecarboxylic acid formic acid 2.A (R=H, Z=Me, A=COOCH 3 ) (10.1 g, 43.7 mmol) and dimethylamino-pyridine (0.1 g). The solution was stirred at 0°C for 1 hour, then at room temperature for 22 hours. The reaction was quenched with water (300 mL) and extracted with dichloromethane. The organic layer was washed with water and MgSO 4 Dry, filter and concentrate. The crude product was crystallized from EtOH to give 2.4a (R=H, L=OTos, A=COOCH 3 ) (13.2 g, 81.6%).
[0137] Melting point 83.1°C;
[0138] IR (KBr): 1734, 1175 cm -1 ;
[0139] 1 H-NMR (CDCl 3 ): δ1.5(3H, m), 2.0(3H, s), 2.28(4H, m), 2.47(3H, s), 3.68(3H...
Embodiment 2
[0175] Example 2: (2S, 3aS, 4aS)-2-tert-Butyldimethylsilyloxy-3a-(hydroxymethyl)-bicyclo[3.1.0]
[0176] Hexane: I.b.2 (R=H, P=TBDMS, A=CH 2 OH).
[0177] A 1.5M solution of diisobutylaluminum hydride in toluene (1.25 L) was added to (2S,3aS,4aS)-2-tert-butyl in toluene (2.1 L) at -70°C over a period of 1.5 hours. Dimethylsilyloxy-3a-methoxy-bicyclo[3.1.0]hexane I.b.1 (R=H, P=TBDMS, A=COOCH 3 ) (207.5 g, 768 mmol) solution. After the addition was complete, a saturated solution of sodium potassium tartrate was added slowly and the temperature was allowed to rise to 0°C. After stirring for 2 hours, the reaction mixture was extracted with toluene, and the organic layer was washed with MgSO 4 Drying and concentration afforded 142.9 g (88%) of the title compound I.b.2 as a yellow oil.
[0178] IR(KBr): 3355, 1471, 1255, 835, 774 cm -1 ;
[0179] 1 H-NMR (CDCl 3 ): δ0(6H, s), 0.32(1H, m), 0.5(1H, m), 0.83(9H, s), 1.16(1H, m), 1.87(5H, m), 3.54(2H, m) , 4.0 (1H, m) ppm. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com