Ciprofloxacin mandelate, ofloxacin mandelate and preparation process thereof
A technology of ofloxacin mandelate salt and ofloxacin mandelate, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] 1. Process
[0019] ①Theoretical basis
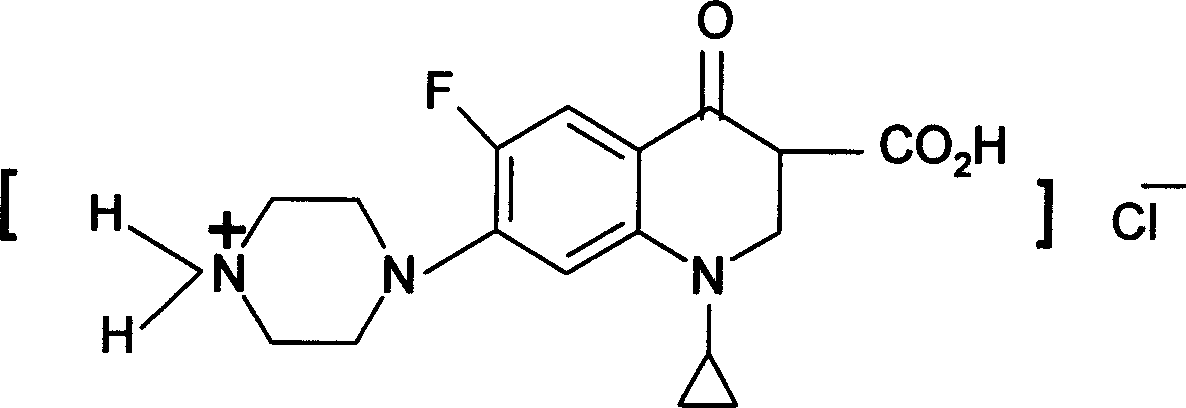
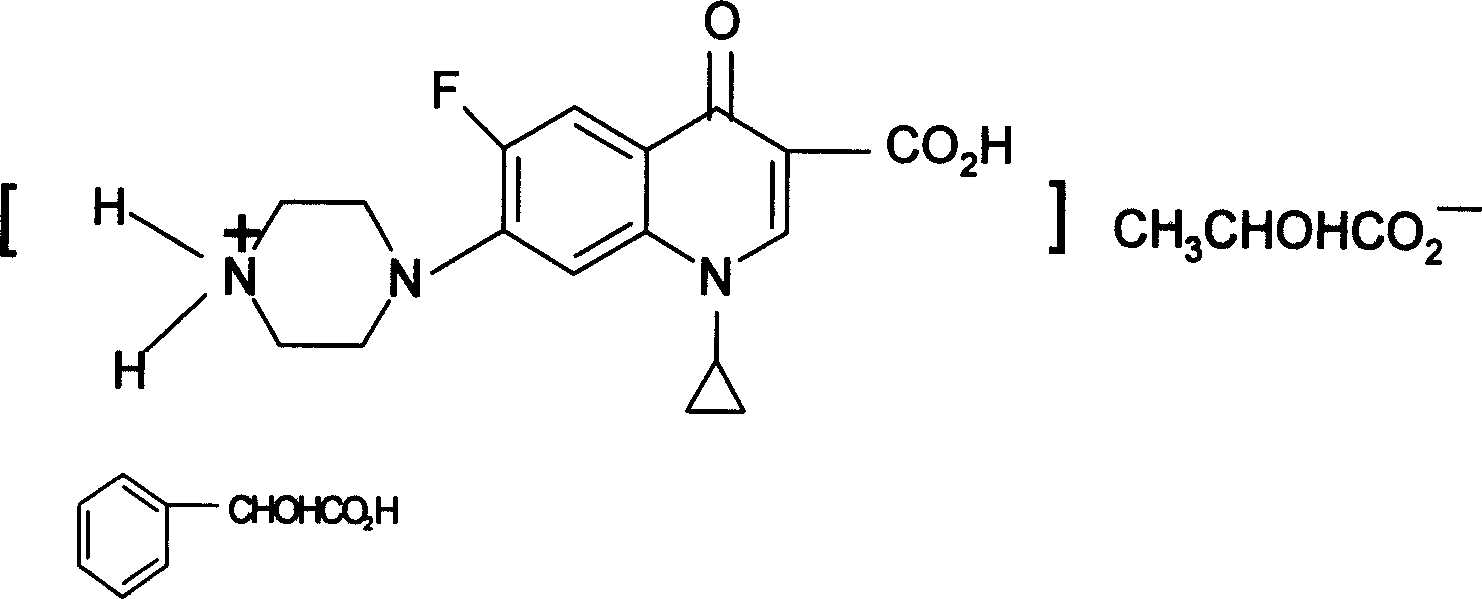
[0020] Both ciprofloxacin and ofloxacin are amphoteric compounds. Its structure has a weakly subtractive tertiary nitrogen atom and a weakly acidic fatty acid carboxyl group. It can exist in the form of an internal salt with low water solubility. In order to increase its water solubility, it can be combined with a strong inorganic acid or a strong organic acid. .
[0021] Mandelic acid is a strong aromatic α-OH group with a pH of 2. The tertiary nitrogen atoms in the structures of ciprofloxacin and ofloxacin can be combined with mandelic acid to form a salt.
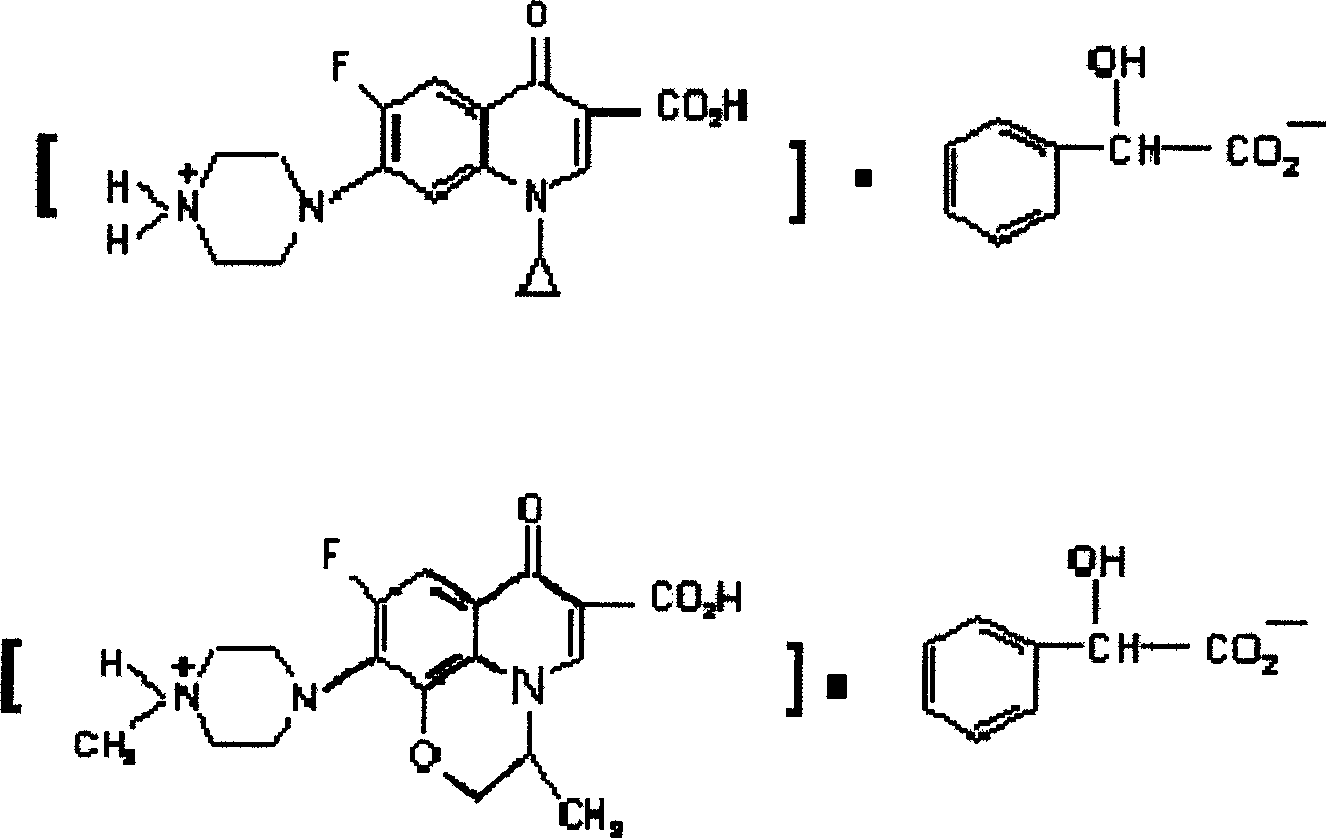
[0022] The reaction formula of ciprofloxacin mandelic acid salt is as follows:
[0023]
[0024] The reaction formula of ofloxacin mandelic acid salt is as follows:
[0025]
[0026] ②Process
[0027] a. Feeding mol ratio
[0028] Mandelic acid: Ciprofloxacin or Ofloxacin = 1.5-2.0:1
[0029] b. Operation process
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com