Method for preparing organic aerogel and carbon aerogel by using normal pressure exsiccation
A technology of carbon aerogels and organic gels, which is applied in the field of preparing organic aerogels and carbon aerogels, can solve the problems of demanding replacement, high cost, and time-consuming preparation cycle, and achieves low equipment requirements and low preparation process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
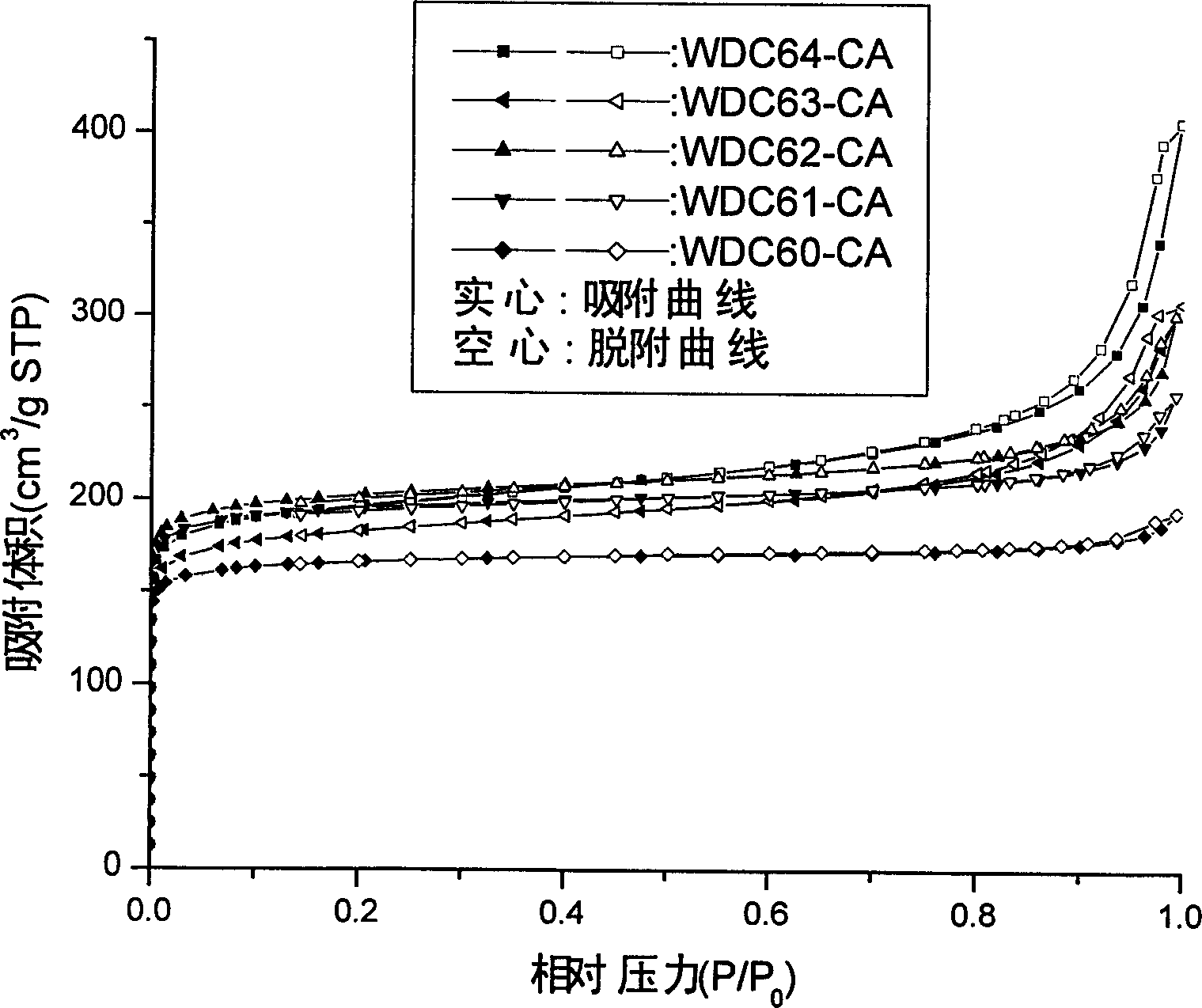
[0016] Embodiment 1: According to R / F=0.5, R / H=25, R / I=0.1g·cm -3 (R / I represents the mass ratio of resorcinol to the volume of isopropanol), 0.0509 gram of hexamethylenetetramine is dissolved in 10 ml of isopropanol, 1 gram of resorcinol is dissolved in 1.5 ml In the furfural, then the two solutions were mixed and stirred evenly, then poured into an ampoule bottle and sealed, and reacted at 75°C for 7 days to obtain an organic gel; then dried at 60°C for 10 hours to obtain an organic aerogel ; Afterwards, place the organic airgel in a desktop carbonization furnace, raise the temperature from room temperature to 900°C at a heating rate of 5°C / min under the protection of high-purity nitrogen, carbonize at a constant temperature for 180 minutes, cool down naturally, and take out the charcoal to condense glue. The measured density of organic airgel is 0.226g cm -3 , with a theoretical density of 0.226g cm -3 Very close; carbon airgel has a density of 0.244 g cm -3 The BET spe...
Embodiment 2
[0017] Embodiment 2: According to R / F=0.5, R / H=45, R / I=0.1g·cm -3 , 0.0283 g of hexamethylenetetramine was dissolved in 10 ml of isopropanol, 1 g of resorcinol was dissolved in 1.5 ml of furfural, the two solutions were mixed and stirred well, and poured into ampoules neutralized and sealed, reacted at 75°C for 7 days to obtain an organic gel; then dried naturally for 3 days and dried at 85°C for about 2 hours (or directly dried at 60°C for 10 hours) to obtain an organic aerogel; after that , put the organic aerogel in a bench-top carbonization furnace, raise the temperature from room temperature to 900°C at a heating rate of 5°C / min under the protection of high-purity nitrogen, carbonize at a constant temperature for 180 minutes, cool down naturally, and take out the carbon aerogel. The measured density of organic airgel is 0.239g cm -3 , with a theoretical density of 0.225g cm -3 Close; the density of carbon airgel is 0.282g cm -3 ; The BET specific surface area of carb...
Embodiment 3
[0018] Embodiment 3: According to R / F=0.5, R / H=50, R / I=0.072g*cm -3 , 0.0254 g of hexamethylenetetramine was dissolved in 13.96 ml of isopropanol, 1 g of resorcinol was dissolved in 1.5 ml of furfural, the two solutions were mixed and stirred well, and then poured into an ampoule and seal it, and react at 75°C for 7 days to obtain an organic gel; then directly dry at 60°C for 10 hours to obtain an organic aerogel; after that, place the organic aerogel in a table-top carbonization furnace, Under the protection of the environment, the temperature was raised from room temperature to 900°C at a heating rate of 5°C / min, carbonized at a constant temperature for 180 minutes, cooled naturally, and the carbon airgel was taken out. The measured density of the organic airgel is 0.238g cm -3 , with a theoretical density of 0.169g cm -3 Close; the density of carbon airgel is 0.223g cm -3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com