Application of RS1 gene in preparation of XLRS therapeutic agent and therapeutic agent
A therapeutic agent, gene technology, applied in the direction of gene therapy, application, genetic engineering, etc., to achieve the effect of improving expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The principles and features of the invention are described below with reference to the accompanying drawings. The examples are only used to explain the invention, but not to limit the scope of the invention.
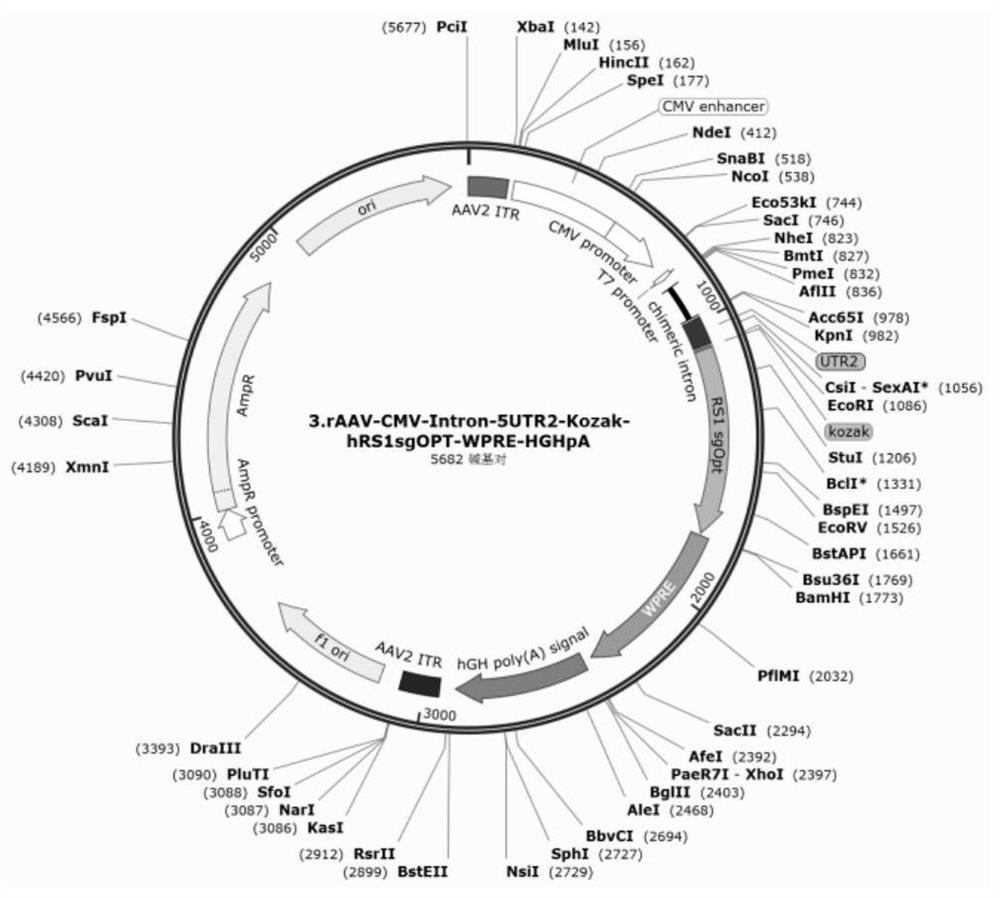
[0024] 1. Construction of AAV-RS1 recombinant expression vector
[0025] In our study, we found that the use of different RS1 expression vectors could have different therapeutic effects on retinoschisis. In order to find the optimal expression vector, we designed three generations of AAV recombinant expression vectors with pAAV-MCS-ITR as the backbone. The first generation is expression vector 1, the second generation is expression vector 2, and the third generation is expression vector 3-5.
[0026] The structures of expression vectors 1 and 2 are as follows figure 1 and 2 As shown, it is rAAV2 / 8-CMV-hRS1-HGHpA, wherein CMV refers to the CMV promoter, the sequence is shown in SEQ ID NO: 1, and HGHpA is human growth hormone polyadenylate, and the sequence is sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com