Lithium isotope separation method and device
A separation method and technology for lithium isotopes, which are applied in separation methods, separation of different isotopic elements, and dispersed particle separation, etc., can solve the problems of solvation effect of convective disturbance ions, affecting the effect of isotope separation, etc., and achieve simple and reliable separation devices. Production cost and the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
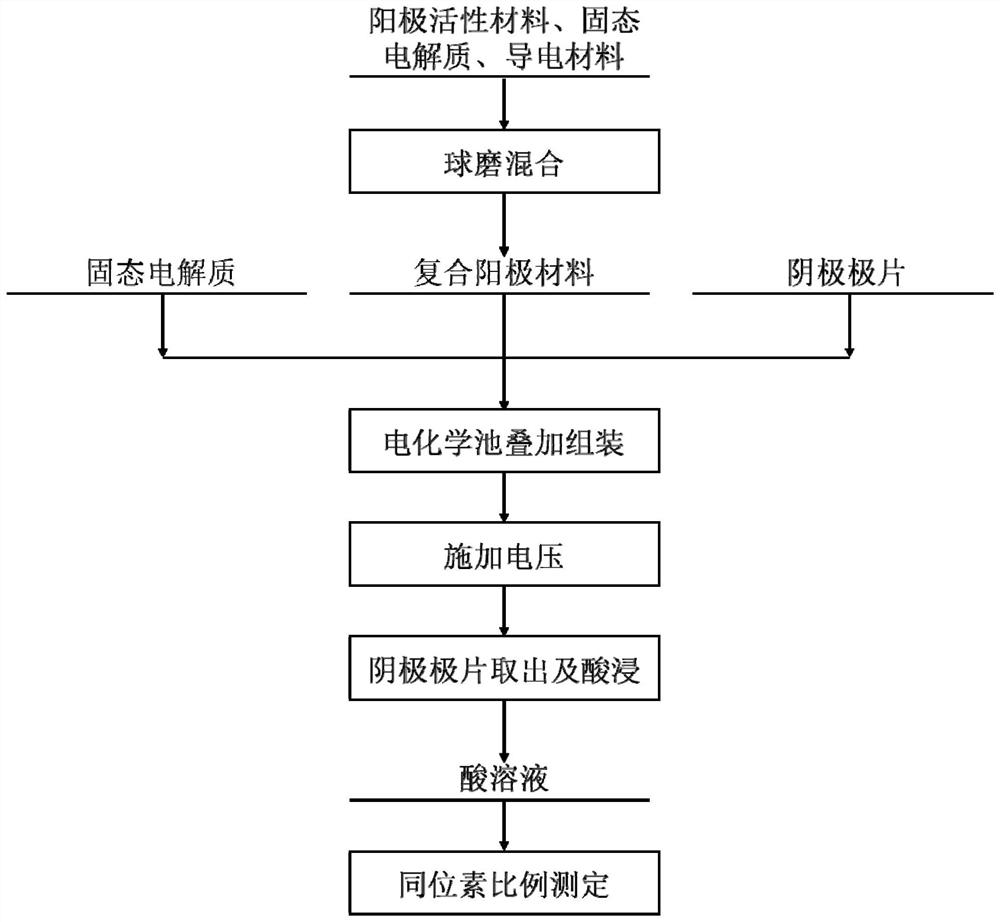
[0032] The nickel-cobalt-manganese ternary lithium, lithium-germanium-phosphorus-sulfur solid-state electrolyte, and Super-P were weighed in a weight ratio of 75:23:2, and ball-milled for 30 minutes to prepare a composite anode material.
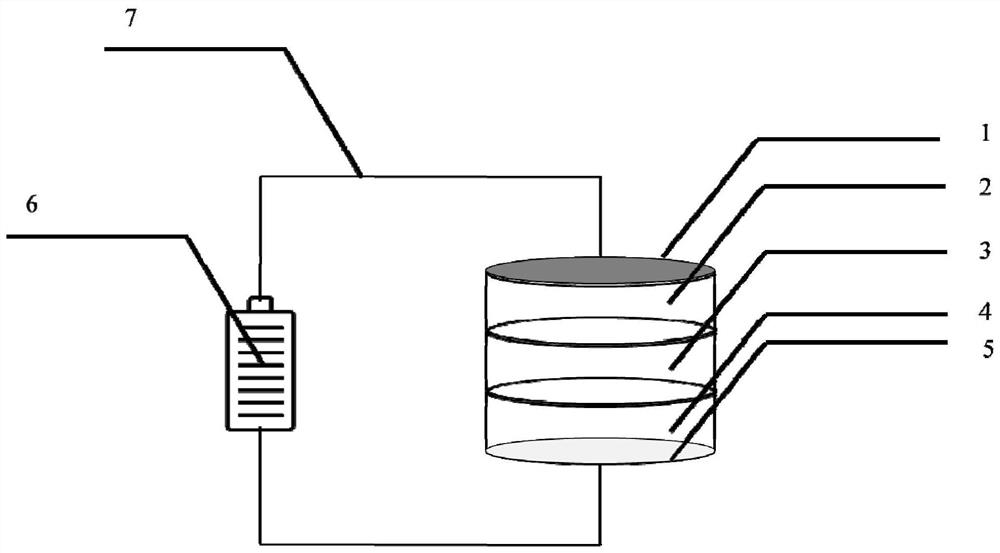
[0033] Weigh 100mg of composite anode material and 800mg of lithium-germanium-phosphorus-sulfur solid-state electrolyte, and place them in a stainless steel mold with a diameter of 1cm in turn. Apply a pressure of 200Mpa for 10min to make the composite anode material and solid-state electrolyte tightly combined. Take a 2mm-thick graphite pole piece As the cathode, it was placed on the other side of the lithium-germanium-phosphorus-sulfur solid electrolyte to assemble a solid-state electrochemical cell. In addition, copper foil and aluminum foil with a thickness of 50 μm were attached to the cathode current collector and the anode current collector in turn. The pressure of 50Mpa was applied for 10min to make each component tightly combine.
...
Embodiment 2
[0039] Lithium cobalt oxide, lithium phosphorus sulfur chloride solid electrolyte and vapor-grown carbon fiber were weighed in a weight ratio of 70:27:3, and ball-milled for 10 minutes to prepare a composite anode material.
[0040] Weigh 11mg composite anode material and 120mg lithium phosphorus sulfur chloride solid electrolyte, place them in a stainless steel mold with a diameter of 1cm in turn, apply a pressure of 370Mpa for 20min, make the composite anode material and solid electrolyte closely combine together, take 2.5mg silver electrode piece as The cathode is placed on the other side of the lithium-phosphorus-sulfur-chlorine solid electrolyte to assemble a solid-state electrochemical cell. The solid-state electrochemical cell is placed in a stainless steel shell of a 2032 button battery, and the pressure of 5Mpa is maintained to make the components tightly combined.
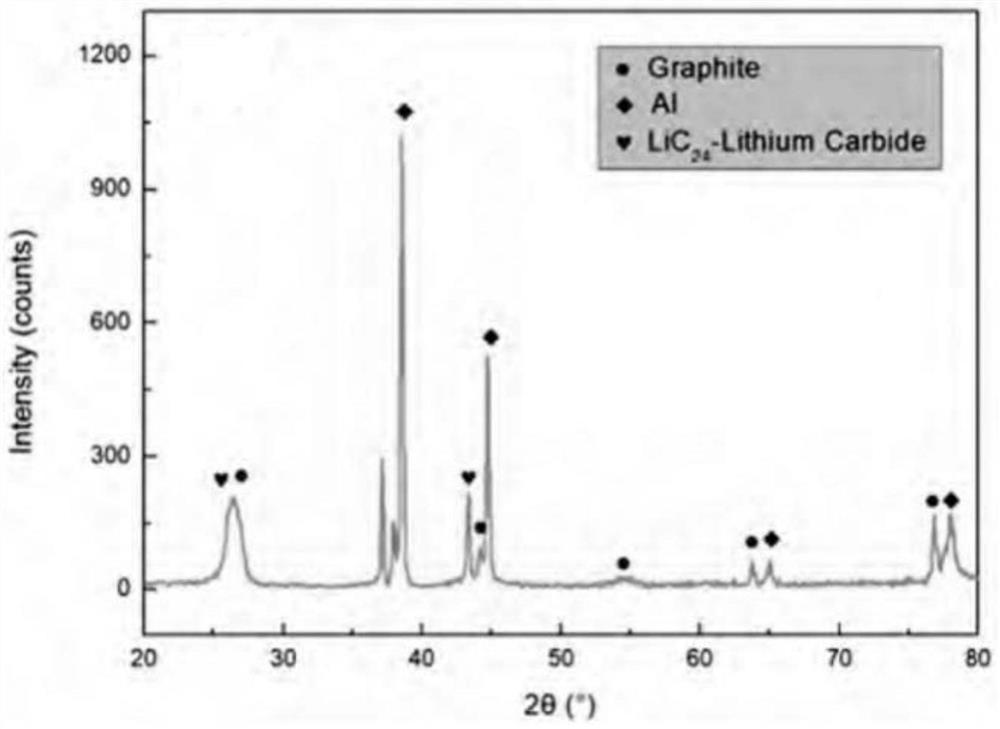
[0041] A voltage of 3.0 V was applied across the solid-state electrochemical cell for 10 min using a Gamr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com