Preparation method of nicardipine hydrochloride injection
A technology for nicardipine hydrochloride and dipine injection, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve the problem of easy production of peroxides in medicinal liquids and lack of attention to stirring atmosphere , Injection quality decline and other issues, to achieve better decolorization effect, good decolorization effect, and safe finished product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of nicardipine hydrochloride injection, comprising the following steps:
[0042] S1. Material preparation: nicardipine hydrochloride with a volume fraction of 0.01%, an adjuvant mixture with a volume fraction of 2%, and the balance of water for injection;
[0043] S2. The adjuvant mixture is 10% of the solubilizer and the balance of the isotonicity regulator;
[0044] S3. after nicardipine hydrochloride and the adjuvant mixture are mixed, water for injection is added to obtain the first treatment solution;
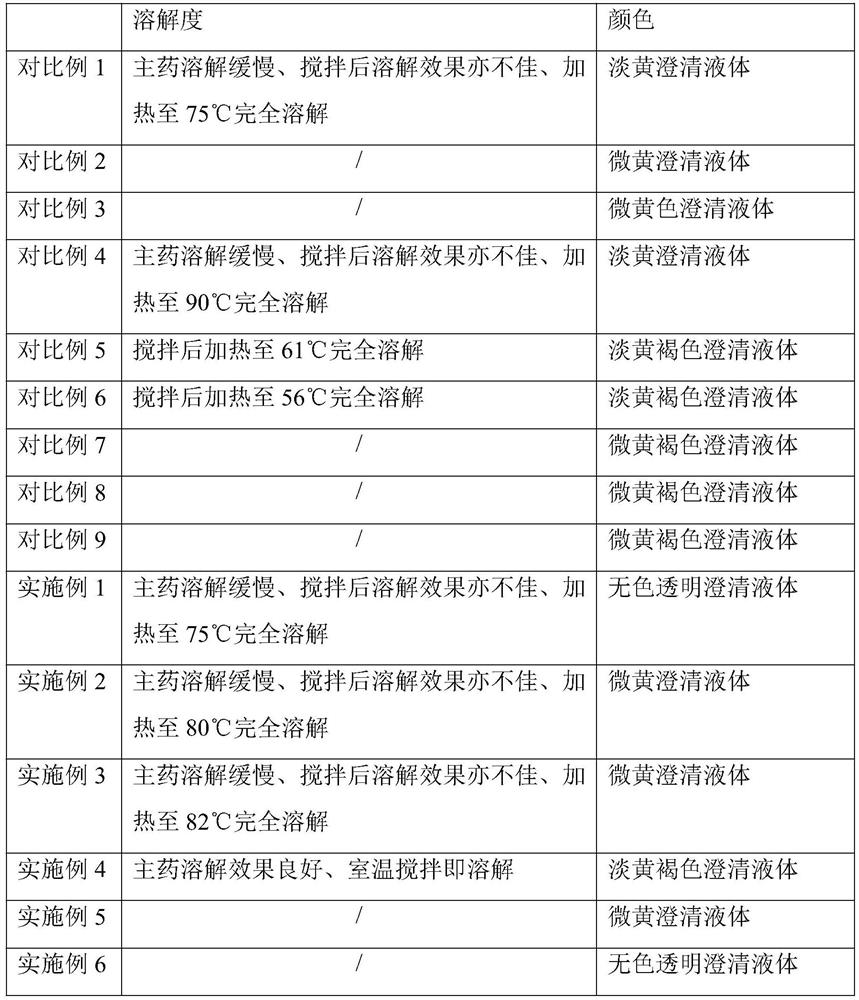
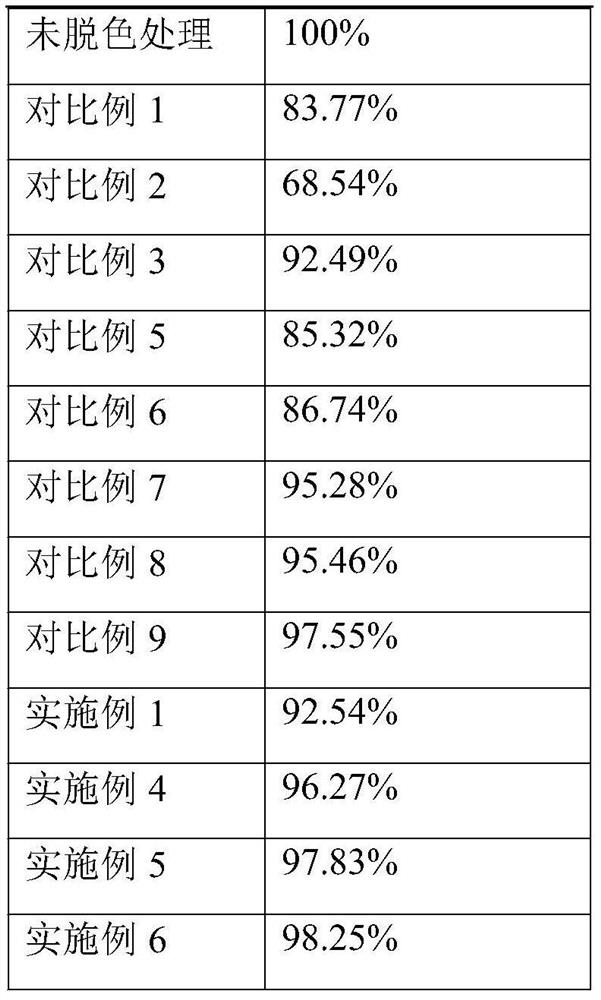
[0045] S4. After the initial treatment of the first treatment solution, under a nitrogen atmosphere, add silica gel modified activated carbon with a total volume of 0.05% to the first treatment solution to stir and decolorize for 25 minutes, and then add silica gel modified activated carbon with a total volume of 0.05% for decolorization with stirring 30min, the second treatment solution was obtained after suction filtration, and the rotating s...
Embodiment 2
[0081] The difference between this embodiment and Embodiment 1 is:
[0082] In the present embodiment, the preparation method of nicardipine hydrochloride comprises the following steps:
[0083] After adding the crude nicardipine hydrochloride into methanol for 50 min of ultrasonic vibration, adding acid solution dropwise to adjust the pH value to 5, and then rotating at 50°C for 4 hours to obtain the first treatment material;
[0084] Add acetone to the first treatment material, cool down to -40°C, react for 1.5h until crystals are completely precipitated, filter out the solid, and dry under reduced pressure at 60°C for 13h to obtain nicardipine hydrochloride;
[0085] The ratio of crude nicardipine hydrochloride to methanol is 1:12;
[0086] The ratio of the first treatment material to acetone was 1:8.
Embodiment 3
[0088] The difference between this embodiment and Embodiment 1 is:
[0089] In the present embodiment, the preparation method of nicardipine hydrochloride comprises the following steps:
[0090] After adding the crude nicardipine hydrochloride into methanol for 50 min of ultrasonic vibration, adding acid solution dropwise to adjust the pH value to 5, and then rotating at 50°C for 4 hours to obtain the first treatment material;
[0091] Add acetone to the first treatment material, cool down to -40°C, react for 1.5h until crystals are completely precipitated, filter out the solid, and dry under reduced pressure at 60°C for 13h to obtain nicardipine hydrochloride;
[0092] The ratio of crude nicardipine hydrochloride to methanol is 1:14;
[0093] The ratio of the first treatment material to acetone was 1:6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com