Klebsiella pneumoniae bacteriophage lyase as well as preparation method and application thereof
A technology of Klebsiella phage and lyase, which is applied in the field of bioengineering, can solve problems such as hindering the contact between lyase and peptidoglycan, and achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of lyase recombinant protein
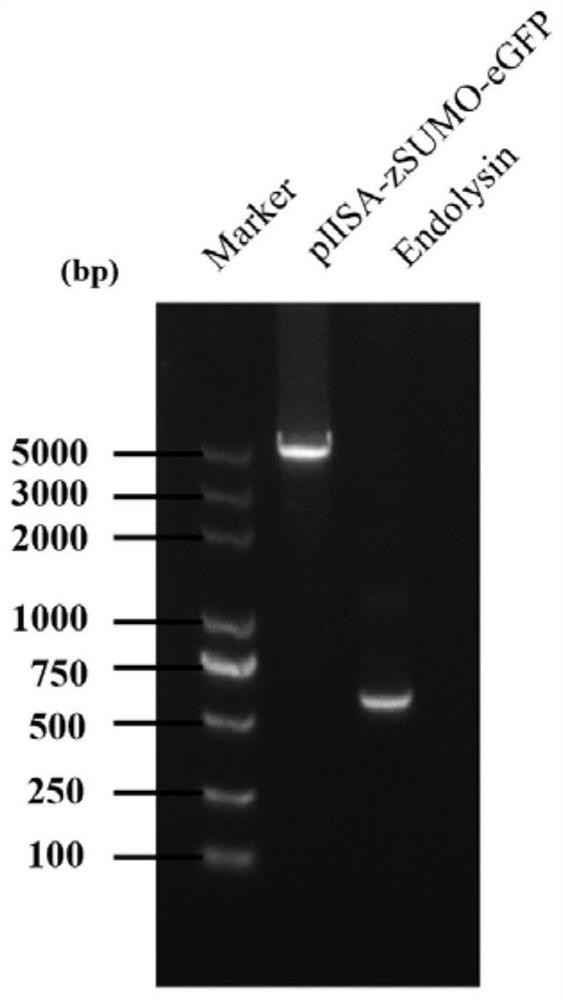
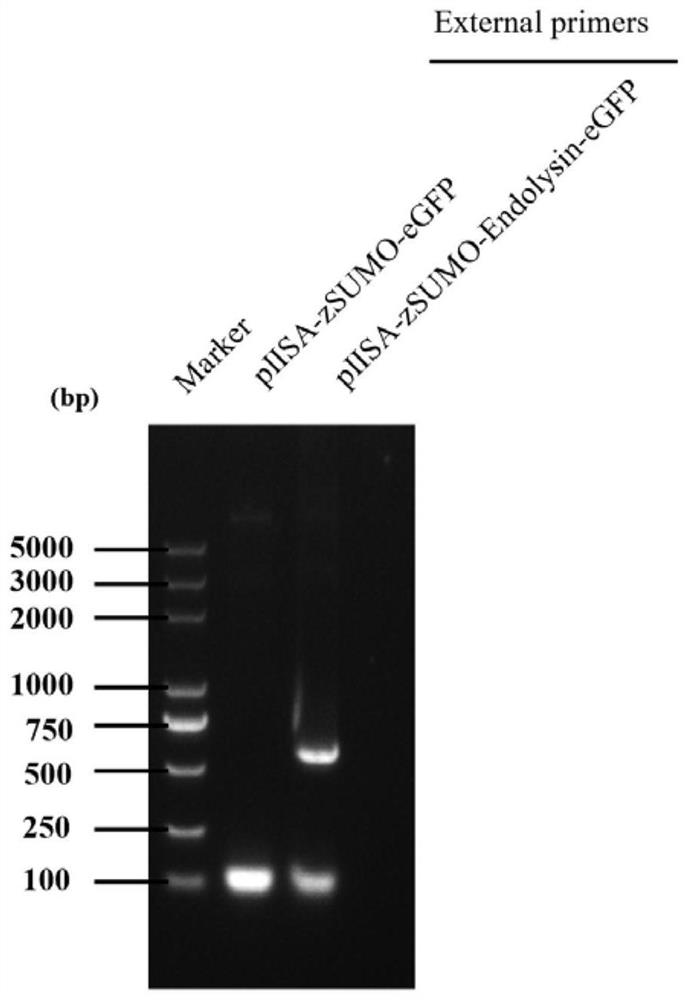
[0036] 1. PCR amplification of lyase encoding gene and prokaryotic expression vector gene
[0037] The PCR amplification of the lyase-encoding gene (nucleotide sequence shown in SEQ ID No. 2), using Klebsiella pneumoniae phage DP genomic DNA as a template, the primer sequence used is: forward primer: aacagattggaggaagcttgaaacttacgctggaacaactcaacaaa (SEQ ID No. .3); Reverse primer: cttgctcaccatagaggttagaacagattttgcctttttgtagtatg (SEQ ID No. 4).
[0038] The PCR amplification of the prokaryotic expression vector gene is based on the prokaryotic expression vector pIISA (which contains the His tag sequence for nickel column purification, the zSUMO tag sequence for improving the stability of the target protein, and the eGFP tag sequence for protein tracking) as Template, the primer sequences used are: forward primer: aaatctgttctaacctctatggtgagcaagggcgagg (SEQ ID No. 5); reverse primer: cgtaagtttcaagcttcctccaatctgttcctgatac...
Embodiment 2
[0048] Example 2 Determination of in vitro antibacterial activity of lyase recombinant protein
[0049] In this example, two typical Gram-negative bacteria, Pseudomonas aeruginosa and Escherichia coli, were selected to verify the bacteriostatic activity of the lyase.
[0050] Escherichia coli in logarithmic growth phase were collected by centrifugation and washed twice with PBS, then resuspended with PBS and adjusted the absorbance OD 600 About 1.0; Recombinant proteins His-zSUMO-Endolysin-eGFP and Endolysin-eGFP with a final concentration of 100 μg / mL were added to each group of bacterial solutions, and His-zSUMO-eGFP was used as the control protein; cultured at 37°C, and then every 30min Measure OD 600 Numeric, plots a curve over time. At the same time, in order to investigate the effect of external membrane penetrant on the bacteriostatic activity of lyase, Triton X-100 (outer membrane penetrant) with a final concentration of 1% was also added to the bacterial solution fo...
Embodiment 3
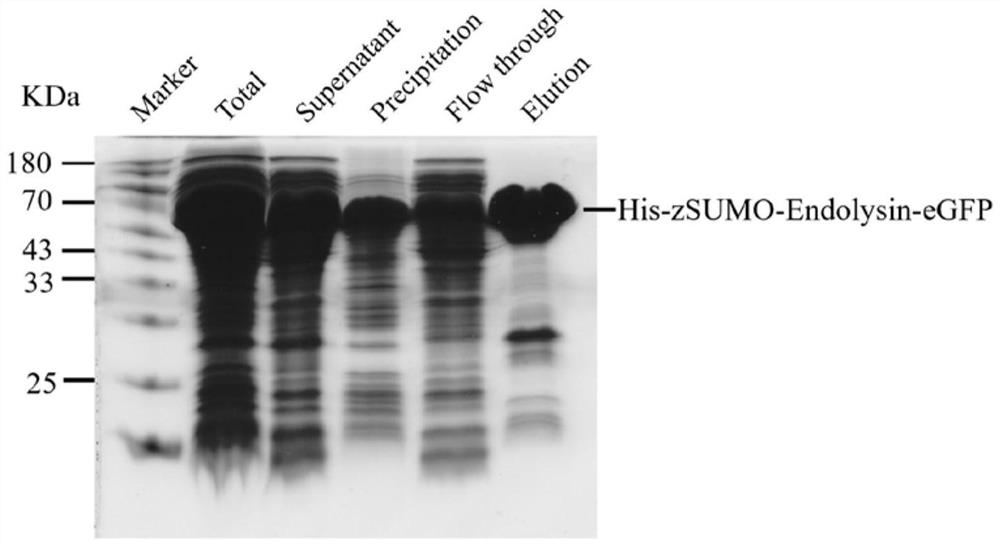
[0053] Example 3 Lyase catalytic active site verification
[0054] Glutamate (E) at positions 53 and 62 of the lyase (amino acid sequence shown in SEQ ID No. 1) was mutated to alanine (A) by site-directed mutagenesis experiments. The mutant plasmid was transformed and the protein was induced to express and purify according to the method described in Example 1 to obtain the lyase mutant protein His-zSUMO-Endolysin-eGFP (E53A, E62A). The SDS-PAGE results are as follows Figure 9 shown.
[0055] The antibacterial activity of the lyase mutant protein His-zSUMO-Endolysin-eGFP (E53A, E62A) was verified by Escherichia coli. The pre-mutated and post-mutated lyase proteins were added to the E. coli solution, and a blank control group was set up and incubated at 37 °C, and OD was measured every 1 h. 600 numerical value. At the same time, the bacteria treated with the protein were counted on the plate, and the difference in the activity of the protein before and after the mutation was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com