Photosensitizer as well as preparation method and application for improving photodynamic performance of photosensitizer
A photosensitizer and reaction technology, applied in the field of photosensitizers, can solve problems such as difficulty in killing specific cancer cells, inability to exert photodynamic effects, and complex composition of nanoparticles, achieving strong tissue penetration, alleviating oxygen consumption, The effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
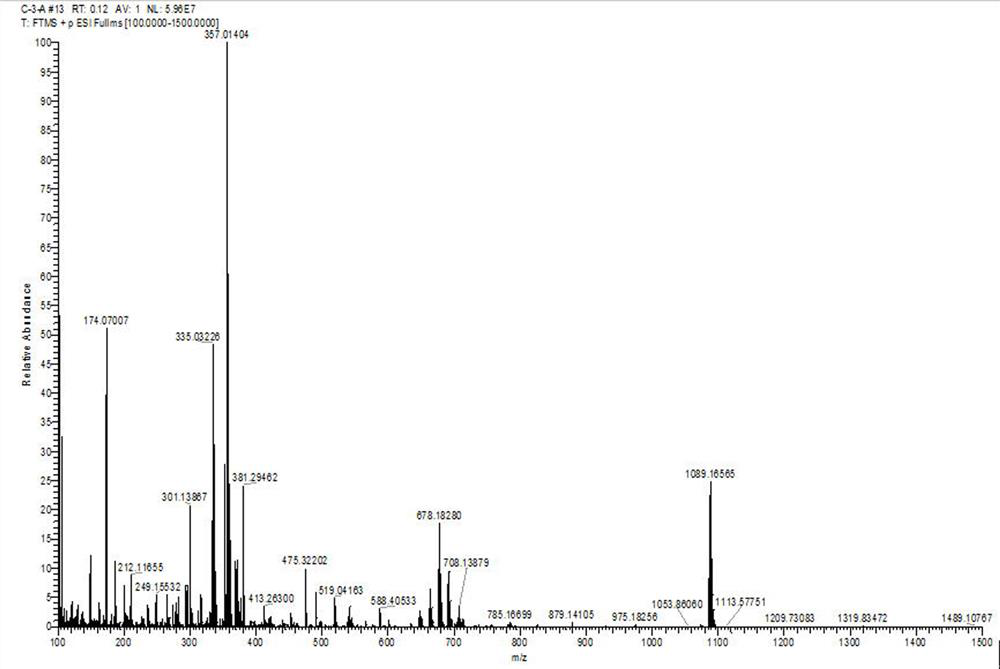
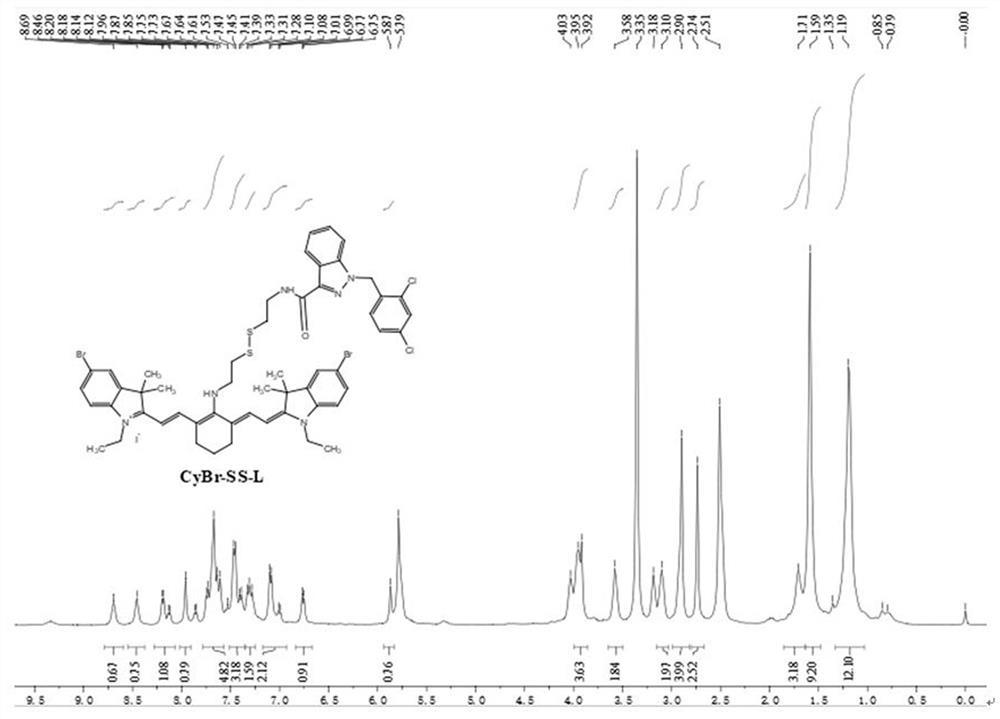
[0070] The preparation method for improving the photodynamic performance of the photosensitizer CyBr-SS-L comprises the following steps:
[0071] (1) Add glacial acetic acid to the reaction flask, mix 4-bromophenylhydrazine hydrochloride and 3-methyl-2-butanone according to the molar ratio of 1:3, and then add it to the reaction flask, the temperature is 110 °C, and the time is 5h, the reaction was completed, the organic phase was extracted with ethyl acetate-water, the ethyl acetate was removed, and the organic layer was spin-dried to obtain product A, namely 5-bromo-2,3,3-trimethyl-3H-indium Indol;
[0072] (2) Prepare product A and ethyl iodide according to the molar ratio of 1:1, first add 5-bromo-2,3,3-trimethyl-3H-indole and ethyl iodide into the reaction flask, dissolve in adjacent In the dichlorobenzene solution, the reaction temperature was 160 °C, the reaction time was 12 h, and the temperature was lowered to room temperature; then suction filtration, washed with et...
Embodiment 2
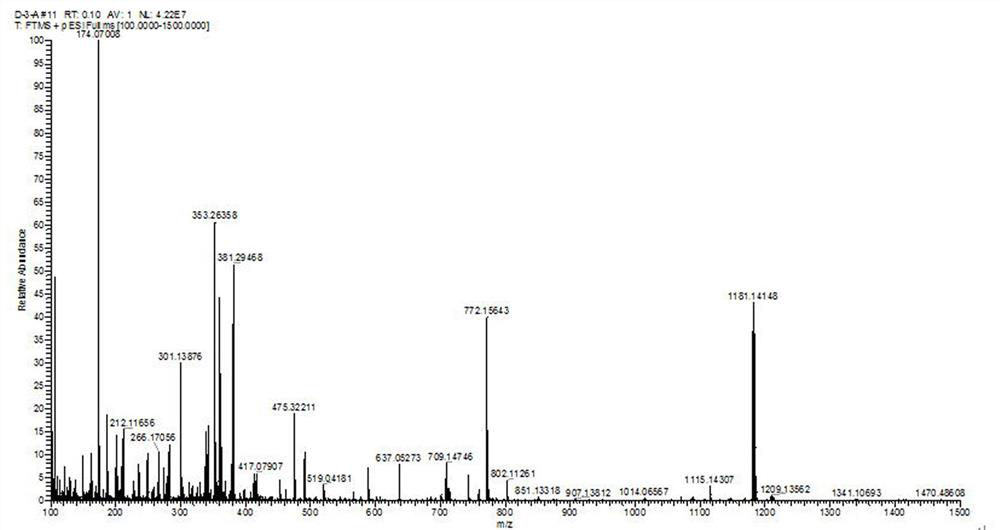
[0086] The preparation method for improving the photodynamic performance of the photosensitizer CyI-SS-L comprises the following steps:
[0087] (1) Add glacial acetic acid to the reaction flask, mix 4-iodophenylhydrazine hydrochloride and 3-methyl-2-butanone according to the molar ratio of 1:2, and then add it to the reaction flask, the temperature is 80 °C, and the time is 12h, the reaction was completed, the organic phase was extracted with ethyl acetate-water, the ethyl acetate was removed, and the organic layer was spin-dried to obtain product A, namely 5-iodo-2,3,3-trimethyl-3H-indium Indol;
[0088] (2) Prepare product A and bromoethane in a molar ratio of 1:2, first add 5-iodo-2,3,3-trimethyl-3H-indole and bromoethane to the reaction flask and dissolve in toluene. In the solution, the reaction temperature was 130°C, the reaction time was 15h, and the temperature was lowered to room temperature; then suction filtration, washed with ethyl acetate, and dried to obtain pr...
Embodiment 3
[0102] The preparation method for improving the photodynamic performance of the photosensitizer CyH-SS-L comprises the following steps:
[0103] (1) Add glacial acetic acid to the reaction flask, mix phenylhydrazine hydrochloride and 3-methyl-2-butanone according to the molar ratio of 1:1, then add it to the reaction flask, the temperature is 100°C, the time is 9h, and the reaction After completion, the organic phase was extracted with ethyl acetate-water, the ethyl acetate was removed, and the organic layer was spin-dried to obtain product A, namely 2,3,3-trimethyl-3H-indole;
[0104] (2) Prepare product A and chloroethane in a molar ratio of 1:3, first add 2,3,3-trimethyl-3H-indole and chloroethane into the reaction flask, dissolve in acetonitrile solution, and react. The temperature was 80 °C, the reaction time was 20 h, and the temperature was lowered to room temperature; then suction filtration, washed with ethyl acetate, and dried to obtain product B, that is, the indole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com