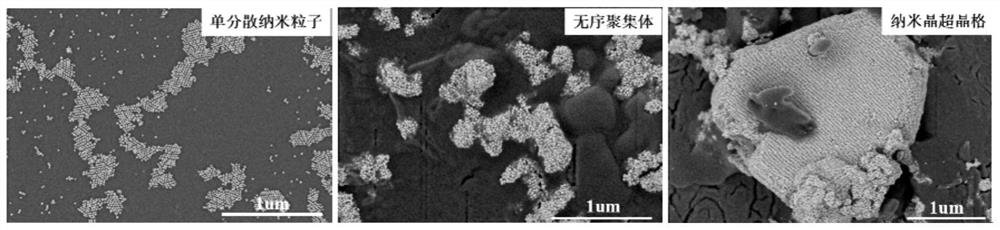
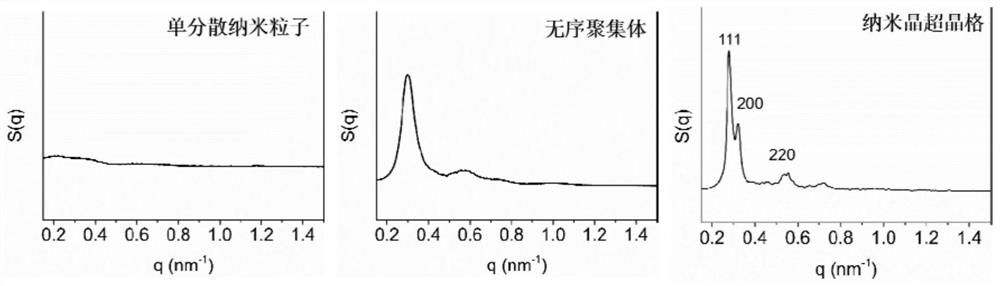
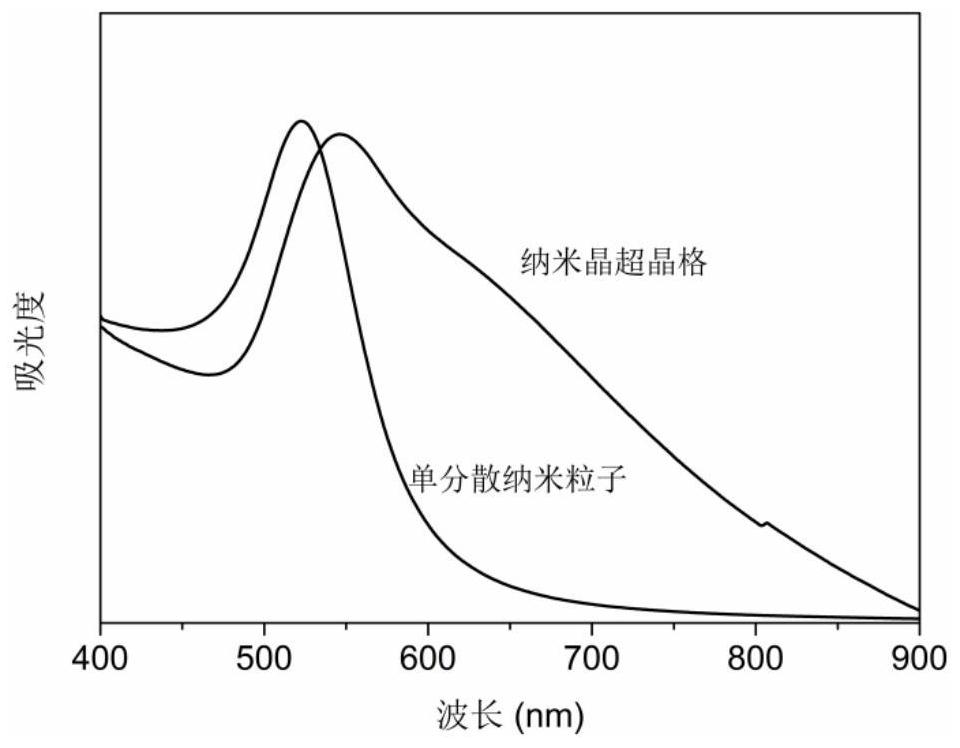
Preparation method of nanocrystalline superlattice material
A nanocrystalline and superlattice technology, applied in metal processing equipment, transportation and packaging, etc., can solve the problems of restricting the industrial application of nanocrystalline superlattice, high requirements for experimental conditions and experimental equipment, and achieve low requirements for experimental equipment , the effect of improving the preparation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] A preparation method of nanocrystalline superlattice material, the steps are as follows:
[0031] (1) Gold nanoparticles, silver nanoparticles, platinum nanoparticles, silica nanoparticles, Ferric oxide nanoparticles or cadmium telluride quantum dots are dispersed in deionized water to obtain a monodisperse nanocrystal aqueous solution with a concentration of 10-50nM;
[0032] (2) adding the inorganic salt (potassium carbonate, sodium carbonate, ammonium carbonate, potassium sulfate, sodium sulfate, ammonium sulfate or magnesium sulfate) solution with a concentration of more than 2M to the monodisperse nanocrystalline aqueous solution obtained in step (1), making the solution The concentration of the inorganic salt in the medium is above 1.0M, and after mixing evenly, let it stand at room temperature (25°C) for 10-20min to obtain disordered aggregates;
[0033] (3) Add deionized water to the disordered aggregates obtained in step (2), so that the inorganic salt concent...
Embodiment 1
[0035] A preparation method of nanocrystalline superlattice material, the steps are as follows:
[0036] (1) A solution of sulfhydryl-modified polyethylene glycol (number average molecular weight 1000) with a concentration of 1 mM was added to a solution of gold nanospheres (average diameter of 20 nm) with a concentration of 1.2 nM, wherein the sulfhydryl-modified polyethylene glycol The molar ratio with gold nanospheres was 5000:1, and after mixing uniformly, the reaction was carried out at 25 °C for 24 h, and the polyethylene glycol-modified gold nanospheres (PEG@NPs) were obtained by ligand exchange;
[0037] (2) centrifugally washing the polyethylene glycol-modified gold nanospheres obtained in step (1) for three times and then dispersing them into deionized water to obtain a monodisperse nanocrystal aqueous solution with a concentration of 20 nM;
[0038] (3) adding a potassium carbonate solution with a concentration of 2M to the monodisperse nanocrystalline aqueous solut...
Embodiment 2
[0050] A preparation method of a nanocrystalline superlattice material is basically the same as that of Embodiment 1, except that the potassium carbonate concentration in the system after adding deionized water in step (4) is 0.83M, and the final obtained nanocrystalline superlattice materials such as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com