Method for synthesizing 2, 4-dichlorotoluene and 3, 4-dichlorotoluene by directionally chlorinating parachlorotoluene with supported catalyst
A supported catalyst, p-chlorotoluene technology, applied in the direction of molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as strong corrosion, polluting the environment, and affecting the service life of devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
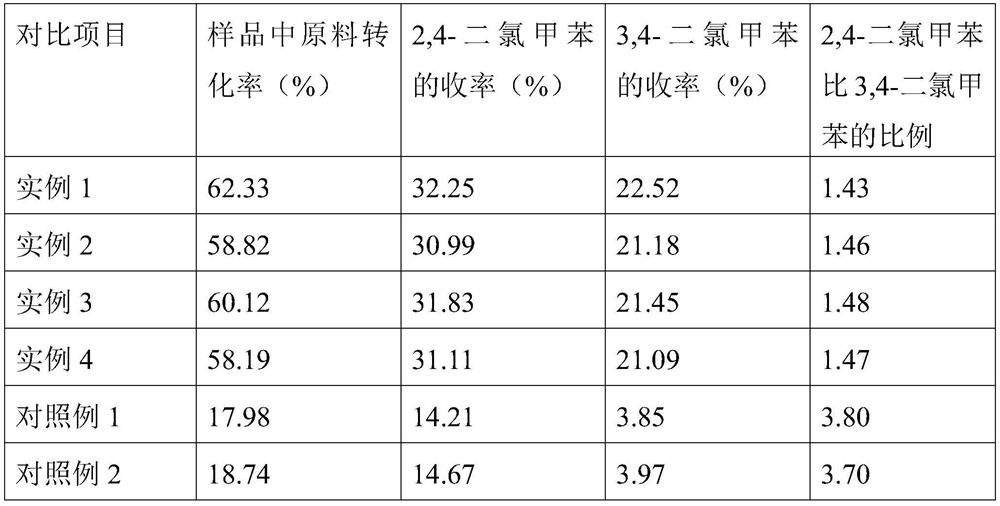
[0036] Take a 500mL four-necked flask, add 95g of p-chlorotoluene, take 3.2g ZSM-5 type molecular sieve supported anhydrous SbCl3 supported catalyst, weigh the ZSM-5 type molecular sieve loaded anhydrous SbCl in the above steps 3 The supported catalyst was added to the above-mentioned four-necked flask; the four-necked flask was placed in an oil bath, and chlorine gas was passed through at a flow rate of 7mL / min and heated to 40°C, and the reaction was stirred for 6h.
[0037] After 6 hours of reaction, samples were taken for analysis. The conversion rate of raw materials in the sample was 62.33%, the yield of 2,4-dichlorotoluene was 32.25%, and the yield of 3,4-dichlorotoluene was 22.52%. The ratio of 2,4-dichlorotoluene to 3,4-dichlorotoluene was The ratio of chlorotoluene was 1.43.
example 2
[0039] Take a 500mL four-necked flask, add 98g of p-chlorotoluene, and take 3g of ZSM-5 molecular sieve-loaded anhydrous SbCl 3 Loaded catalyst, the 3g ZSM-5 molecular sieve-loaded anhydrous SbCl that was weighed in the above steps was added into the above-mentioned four-necked flask; the four-necked flask was placed in an oil bath, and the flow rate was 8mL / min. Chlorine was passed through and heated to 41 °C, and the reaction was stirred for 6.5 h.
[0040] After 6.5 hours of reaction, samples were taken for analysis. In the sample, the conversion rate of raw materials was 58.82%, the yield of 2,4-dichlorotoluene was 30.99%, and the yield of 3,4-dichlorotoluene was 21.18%. The ratio of 2,4-dichlorotoluene to 3,4-dichlorotoluene was The ratio of chlorotoluene was 1.46.
example 3
[0042] Take a 500mL four-necked flask, add 100g of p-chlorotoluene, get 3g ZSM-5 type molecular sieve-loaded anhydrous SbCl The supported catalyst, the 3g ZSM-5 type molecular sieve-loaded anhydrous SbCl that was taken in the above steps The supported catalyst was added to the above-mentioned In a four-necked flask; put the four-necked flask in an oil bath, pass chlorine gas at a flow rate of 9 mL / min, heat to 42° C., and stir for 6 h.
[0043] After 6 hours of reaction, samples were taken for analysis. The conversion rate of raw materials in the sample was 60.12%, the yield of 2,4-dichlorotoluene was 31.83%, and the yield of 3,4-dichlorotoluene was 21.45%. The ratio of 2,4-dichlorotoluene to 3,4-dichlorotoluene was The ratio of chlorotoluene was 1.48.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com