Nucleoside compound and application thereof in treating feline infectious peritonitis
A compound and drug technology, applied to nucleoside compounds and their application in the treatment of feline infectious peritonitis, can solve the problems of large side effects and can not reduce the high fatality rate of the disease and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of Compound 32:
[0057]
[0058] Step 1: 1.32 g of tert-butoxyacetic acid and 0.84 g of sodium bicarbonate were added to 30 mL of DMSO, and stirred at room temperature for 20 min until dissolved. 2.0 g of raw material A1 was added at room temperature, and the temperature was increased to 65° C. to react overnight. The raw material disappeared and the product was formed. The reaction was stopped, extracted with EA / water until the aqueous layer was substantially free of product, the organic phase was washed with saturated brine, dried and concentrated on the column, eluted with DCM / MeOH=30 / 1 to obtain 1.1 g of white solid B1.
[0059] 1 H NMR (400MHz, DMSO-d 6)δ8.05–7.86(m,3H),6.92(d,J=4.4Hz,1H),6.82(d,J=4.4Hz,1H),6.36(d,J=6.0Hz,1H),5.42( d, J=5.8Hz, 1H), 4.68(t, J=5.4Hz, 1H), 4.38(d, J=9.8Hz, 1H), 4.28–4.14(m, 2H), 4.00(s, 2H), 3.96–3.89(m, 1H), 1.12(s, 9H).
[0060] Step 2: 500 mg of the starting compound B1 was dissolved in 10 mL of DCM,...
Embodiment 2
[0086] Example 2: Solubility Test
[0087] Experimental method: refer to high performance liquid chromatography (Chinese Pharmacopoeia 2020 edition of the four general rules 0512).
[0088] a. Take about 1-2 mg of the compound, put it in a 10 ml volumetric flask, add 1 ml of water, shake vigorously for 30 seconds every 5 minutes, observe for 30 minutes, the sample is not completely dissolved, filter through a 0.45 μm filter membrane, and take the continuous filtrate as Test solution.
[0089] b. Take another appropriate amount of reference substance, dissolve in solvent (DMSO:methanol=10:90) and quantitatively dilute to make a solution containing 0.1mg per 1ml, as reference substance solution.
[0090] c. Precisely measure 20 μl of the reference substance and the test solution, respectively, inject them into the liquid chromatograph, and calculate the peak area according to the external standard method. The data obtained are shown in Table 2. Calculation formula: In the fo...
Embodiment 3
[0097] Example 3: Efficacy test of compounds 1, 32 and 85 on feline infectious peritonitis
[0098] experimental method:
[0099] 15 mg of compounds 1, 32, and 85 were dissolved in 5 mL of a solution (water:propylene glycol:PEG=2:1:1), respectively, to prepare an injection solution and administered by injection. The injection solution can be stored at room temperature. Use a 2mL disposable veterinary syringe to draw the corresponding volume of the injection solution and remove the air upwards. When injecting, choose a place with soft skin, loose subcutaneous tissue and less blood vessels, such as subcutaneous injection in the neck or medial thigh. The dosage is 2mg / Kg body weight once a day, blood is collected every three days and blood routine and corresponding biochemical index determination are carried out. For each compound, 15 cats with confirmed feline infectious peritonitis were tested.
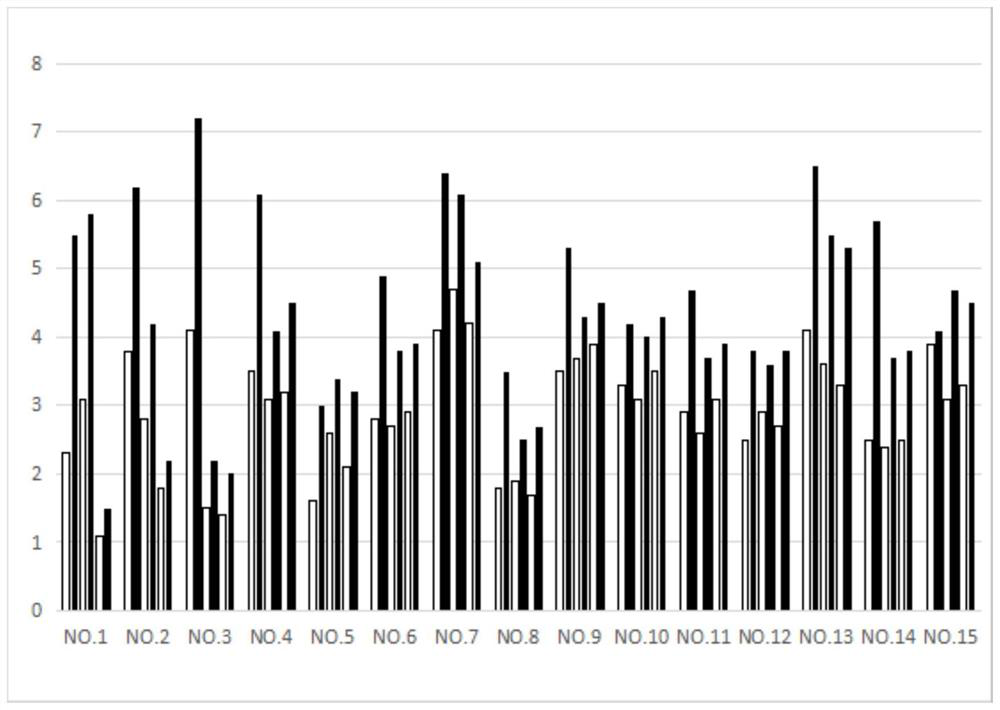
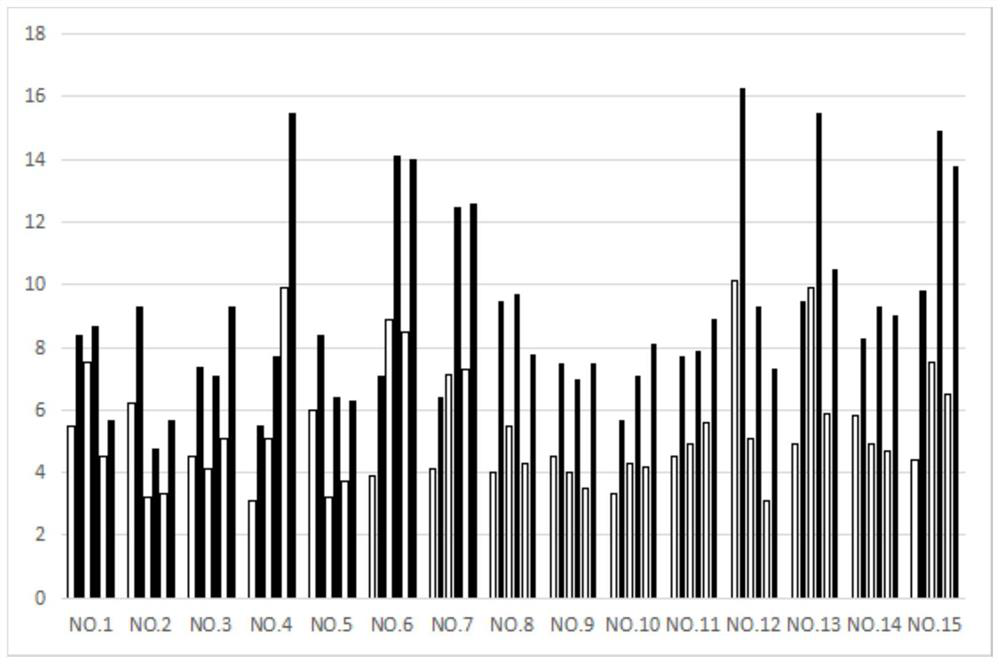
[0100]figure 1 Shows the body weight changes of 15 cats in each of the 3 groups...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com