Method for determining content of tiamulin and valnemulin in veterinary drug preparation through solid phase extraction-high performance liquid chromatography-tandem mass spectrometry
A high-performance liquid chromatography, tiamulin technology, applied in the field of drug testing, can solve problems such as infection, antibiotic residue hazards, flora imbalance, etc., and achieve the effects of improving work efficiency, good linear relationship, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] To prepare standard working solution:
[0076] Precisely weigh 10 mg of tiamulin and vornimulin respectively (accurate to 0.01 mg), dissolve in acetonitrile and dilute to 100 mL, and prepare a standard stock solution with a mass concentration of 100 mg / L; accurately pipette 1 mL of each The above stock solution was placed in a 100mL volumetric flask, and the mixed standard intermediate solution of 1.0 mg / L was prepared with acetonitrile; L, 5μg / L, 10μg / L, 20μg / L, 50μg / L, 100μg / L standard working solutions.
[0077] Sample pretreatment:
[0078] Weigh 1.0 g of sample (accurate to 0.01 g) into a 50 mL centrifuge tube with a stopper, add 20 mL of acetonitrile, cover and mix well, vortex on a vortex shaker for 5 min, and centrifuge at 4000 r / min for 5 min after extraction.
[0079] A MCX solid phase extraction cartridge (60 mg, 3 mL) was activated with 3 mL of methanol and equilibrated with 3 mL of 0.1% aqueous formic acid. Take 2mL of the above-mentioned acetonitrile ex...
experiment example 1
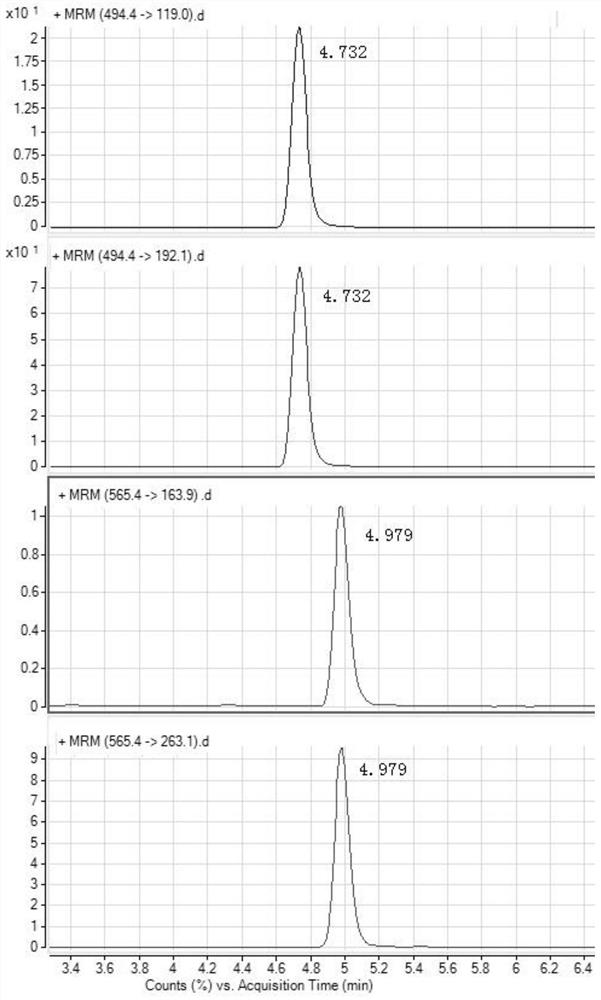
[0088] Chromatographic separation and mass spectrometry detection of the mixed solution of the analyte:
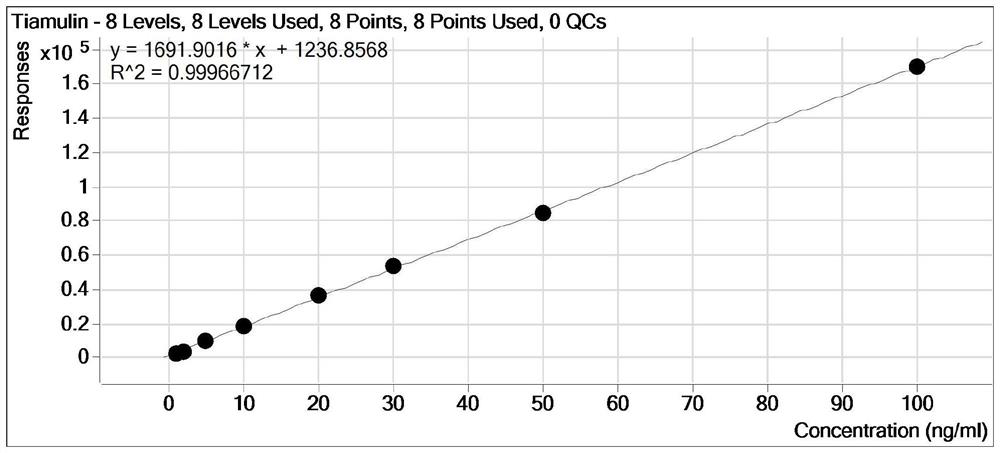
[0089] Method Linearity, Limits of Detection, and Limits of Quantitation
[0090] Under the experimental conditions determined by this method, the preparation concentrations were 1.0ng / mL, 2.0ng / mL, 5.0ng / mL, 10.0ng / mL, 20.0ng / mL, 30.0ng / mL, 50.0ng / mL, 100.0 Eight standard series of ng / mL were measured by LC / MS, and the response peak area (Y-axis) of quantitative ions was plotted against the corresponding mass concentration (X-axis, ng / mL). The results are shown in Table 3. The results showed that in the range of 1.0-100 ng / mL for tiamulin and vonimulin, there was a good linear relationship between the response peak area of the quantitative ion and the sample concentration, and the correlation coefficients were all greater than 0.99. See image 3 and Figure 4 .
[0091] According to the sample amount represented by the final sample solution, the volume of constant vo...
experiment example 2
[0096] Matrix effect detection
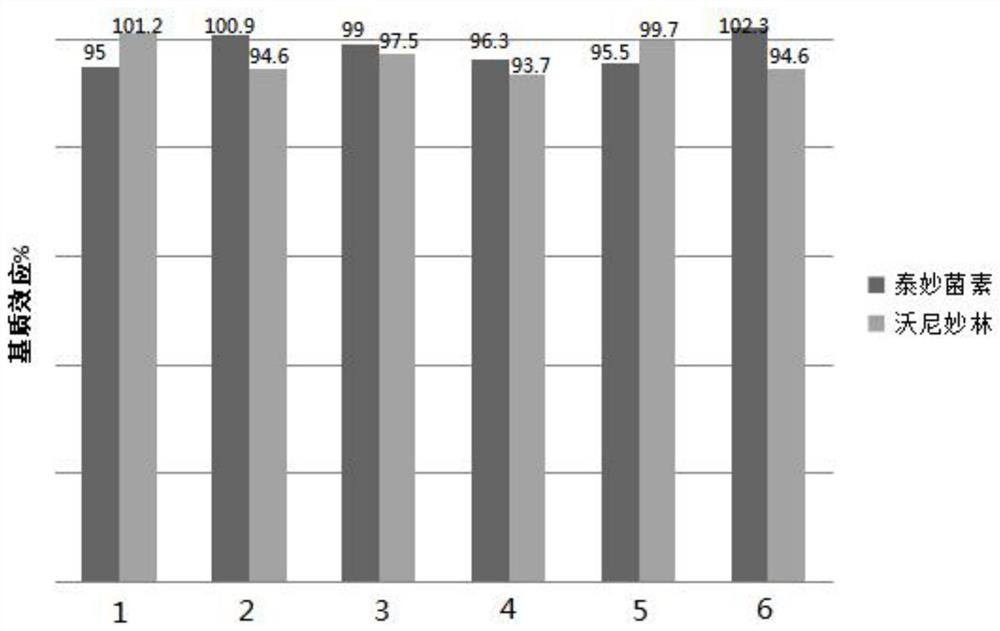
[0097] Matrix effects (ME) refer to the ionization effect of co-eluted substances during chromatographic separation, resulting in the inhibition or enhancement of the signal. When the influence of matrix effect is large, it will reduce the sensitivity of the method and affect the accuracy of the method, which will bring errors to the determination. Therefore, it is necessary to evaluate the matrix effects (ME) in the process of liquid chromatography-mass spectrometry method development and confirmation. Matrix effect (%) ME = (slope of matrix-matched standard curve / slope of pure solvent standard curve) x 100%. It is generally believed that the matrix effect is not obvious when the matrix effect is between 85% and 115%. In this method, the standard working curve and matrix matching calibration curve are prepared, the peak area is taken as the ordinate and the mass concentration is plotted as the abscissa. figure 2 It shows that for the three ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com