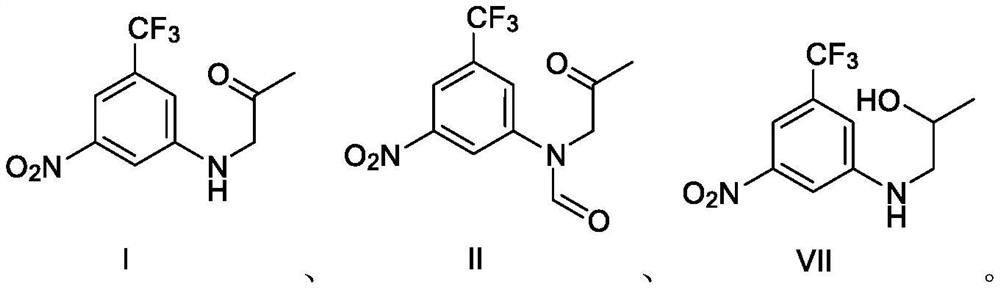
Synthesis method of 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl) aniline and intermediate of 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl) aniline
A technology of triethylamine and diisopropylethylamine, which is applied in the field of synthesizing nilotinib, can solve the problems of low yield and isomer production, and achieve the effects of high yield, mild conditions and many steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1 compound III 3-bromo-5-trifluoromethylaniline
[0056]
[0057] Under the protection of inert gas at room temperature, 3.0 g of compound VI, 15 ml of ethylene glycol, 0.10 g of cuprous oxide, and 8 ml of concentrated ammonia water were added to the sealed reaction system, and heated to 100° C. to react for 48 hours. TLC detected the completion of the reaction, concentrated most of the ethylene glycol, cooled to room temperature, added 50 ml of water, extracted three times with ethyl acetate, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered and concentrated, and dried to obtain 2.1 g of compound III with purity 95.5%, yield 93.8%.
Embodiment 2
[0058] The preparation of embodiment 2 compound III 3-bromo-5-trifluoromethylaniline
[0059]
[0060] Under the protection of inert gas at room temperature, 2.3 g of compound VI, 15 ml of ethylene glycol, 0.10 g of cuprous oxide, and 8 ml of ammonia methanol were added to the sealed reaction system, and the reaction was heated to 100° C. for 12 hours. TLC detected the completion of the reaction, concentrated most of the ethylene glycol, cooled to room temperature, added 50 ml of water, extracted three times with ethyl acetate, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered and concentrated, and dried to obtain 1.51 g of compound III, The purity is 98.1%, and the yield is 86.0%.
Embodiment 3
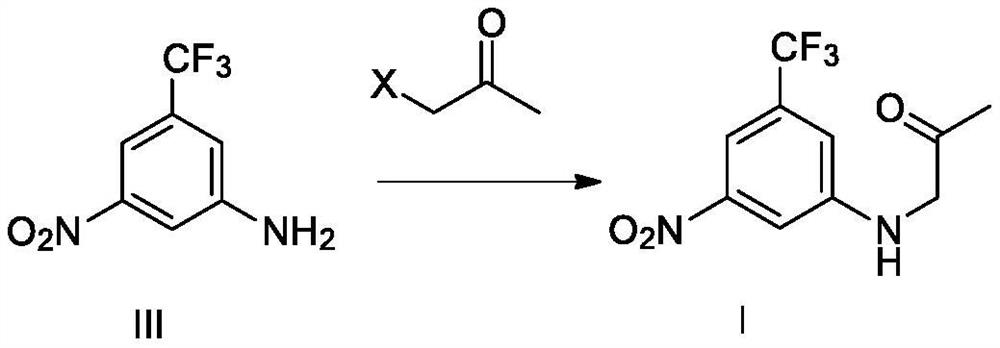
[0061] Example 3 Preparation of Compound I N-(2-oxopropyl)-3-nitro-5-trifluoromethylaniline
[0062]
[0063] Under the protection of inert gas at room temperature, 100 ml of N,N-dimethylformamide, 5.0 g of compound III, 6.7 g of potassium carbonate, and 5.0 g of bromoacetone were sequentially added to the reaction flask, and the temperature was raised at 95° C. to react for 20 hours. The temperature was lowered to room temperature, 100 ml of water was added, and the mixture was extracted three times with 100 ml of ethyl acetate. The organic phases were combined, washed with saturated sodium chloride solution, dried over sodium sulfate, filtered, and concentrated under reduced pressure to remove the organic solvent. 100 ml of petroleum ether was added to the residue, stirred at room temperature for 2 hours, filtered to obtain a crude product of compound I, and dried to obtain 5.4 g of compound I with a purity of 97.3% and a yield of 85.1%.
[0064] MS+[M+1]263,
[0065] 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com