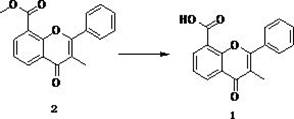
Preparation method of 3-methyl flavone-8-carboxylic acid
A technology of methyl flavonoid and carboxylic acid, applied in the field of pharmaceutical synthesis, can solve the problems of high toxicity, high risk, long synthesis route and the like, and achieves the effects of avoiding expensive price, reducing the generation of isomer impurities, and efficient synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
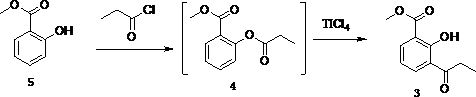
[0033] The first step, the preparation of methyl 3-propionyl salicylate
[0034] Add 30.4g (0.2mol) methyl salicylate and 200ml carbon disulfide to a 500ml four-necked flask, turn on stirring, slowly drop in 20.4g (0.24mol) of propionyl chloride after the solids are all dissolved, heat up to 40°C, and keep the reaction 4h, add 38.0g (0.2mol) of titanium tetrachloride and stir for 0.5h, evaporate the solvent under reduced pressure, heat up to 120 °C for 4h reaction, add ice water to quench the reaction, and distill the oily substance under reduced pressure to obtain 3-propionylsalicylic acid Methyl ester 33.6g, yield 81%.
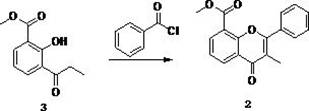
[0035] The second step, the preparation of methyl 3-methylflavonoid-8-carboxylate
[0036] Add 33.0g (0.16mol) methyl 3-propionyl salicylate, 63.9g (0.45mol) benzoyl chloride in turn to the 1000ml reaction flask, turn on stirring, add 64.8g (0.45mol) sodium benzoate, heat up to 170°C The reaction was carried out for 4 hours. Using a solution with a volume...
Embodiment 2
[0040] The first step, the preparation of methyl 3-propionyl salicylate
[0041] Add 30.4g (0.2mol) methyl salicylate and 300ml dichloromethane to a 500ml four-necked flask, turn on stirring, slowly drop in 20.4g (0.24mol) of propionyl chloride after all the solids are dissolved, heat up to 40°C, Incubate the reaction for 4 h, add 38.0 g (0.2 mol) of titanium tetrachloride, stir for 0.5 h, evaporate the solvent under reduced pressure, heat up to 150 °C for 4 h, add ice water to quench the reaction, and distill the oil under reduced pressure to obtain 3-propionyl water 31.1 g of methyl succinate, yield 75%.
[0042] The second step, the preparation of methyl 3-methylflavonoid-8-carboxylate
[0043] Add 30.0g (0.15mol) methyl 3-propionyl salicylate, 59.9g (0.42mol) benzoyl chloride to the 1000ml reaction flask in turn, turn on stirring, add 60.8g (0.42mol) sodium benzoate, heat up to 180°C The reaction was carried out for 4 hours, and a solution with a volume ratio of dichloro...
Embodiment 3
[0047] The first step, the preparation of methyl 3-propionyl salicylate
[0048] Add 30.4g (0.2mol) methyl salicylate and 200ml tetrahydrofuran to a 500ml four-necked flask, turn on stirring, slowly drop in 20.4g (0.24mol) of propionyl chloride after all the solids are dissolved, heat up to 40°C, and heat the reaction 4h, add 26.6g (0.2mol) aluminum trichloride, stir for 0.5h, evaporate the solvent under reduced pressure, heat up to 180 °C for 4h, add ice water to quench the reaction, and distill the oily substance under reduced pressure to obtain 3-propionylsalicylic acid Methyl ester 26.9g, yield 65%.
[0049] The second step, the preparation of methyl 3-methylflavonoid-8-carboxylate
[0050] Add 30.0g (0.15mol) methyl 3-propionyl salicylate and 59.9g (0.42mol) benzoyl chloride to the 1000ml reaction flask in turn, turn on stirring, add 60.8g (0.42mol) sodium benzoate, heat up to 170°C The reaction was carried out for 4 hours, and a solution with a volume ratio of dichloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com