Method for preparing 2, 5-dimethylfuran by catalyzing 2, 5-furandimethanol
A technology for furandimethanol and dimethylfuran, which is applied in the field of preparing 2,5-dimethylfuran, can solve the problems of hydrogen limitation, reduced safety factor, inconvenience and the like, achieves improvement of selectivity and yield, avoids high temperature and high pressure Condition, effects on storage and transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
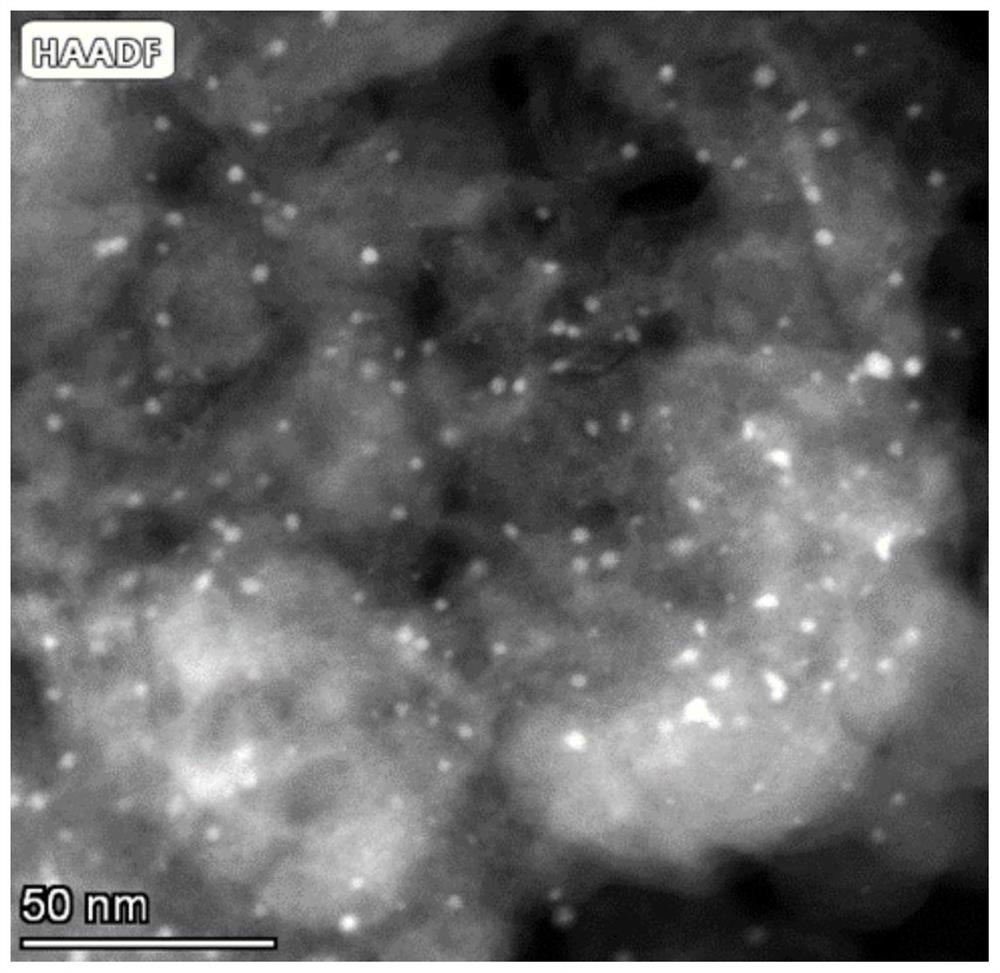
Image
Examples
Embodiment 1
[0065] (1) Add 0.5g XC-72, 45mL deionized water, 5mL ethanol to the beaker, add 0.235mmol Na after sonicating for 40min 2 PdCl 4 , and stirred at room temperature for 6h. Then, 5 mL of freshly prepared 0.2 mol / L sodium borohydride solution was added for reduction for 2 h, and the Pd / XC-72 catalyst was obtained by washing.
[0066] (2) Take 0.26g of 2,5-furandimethanol and add it to 15mL of dioxane to prepare a reaction substrate solution. Combine the reaction substrate solution, 0.76mL of anhydrous formic acid and 0.2g of the prepared solution in (1). The Pd / XC-72 catalyst was mixed and placed in the reaction kettle, the gas in the kettle was replaced with nitrogen five times, the air was exhausted, and the reaction was heated to 120 °C at a stirring rate of 400 r / min for 15 h to obtain the product 2,5-dimethylfuran.
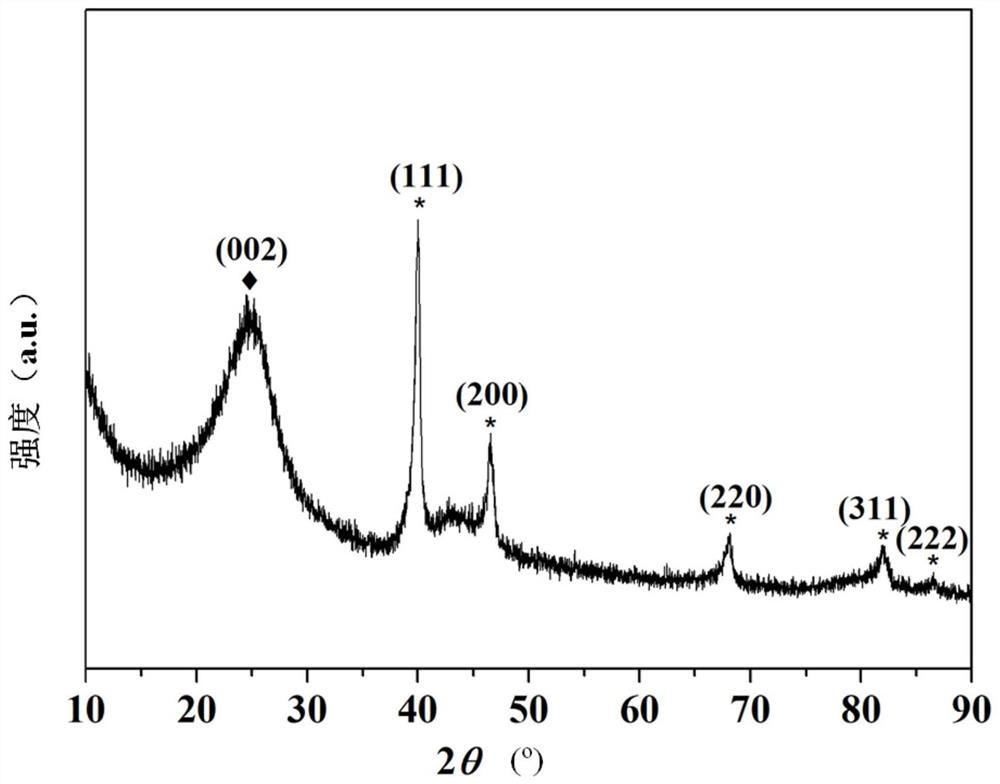
[0067] like figure 1 Shown is the XRD pattern of the prepared Pd / XC-72 catalyst, which has an obvious diffraction peak at the 2-theta angle of 25.0 (002), wh...
Embodiment 2
[0077] (1) Add 0.5g CNT, 45mL deionized water, 5mL ethanol to the beaker, add 0.235mmolNa after sonicating for 40min 2 PdCl 4 , and stirred at room temperature for 6h. Then, 5 mL of freshly prepared 0.2 mol / L sodium borohydride solution was added for reduction for 2 h, and the Pd / CNT catalyst was obtained by washing.
[0078] (2) Take 0.26g of 2,5-furandimethanol and add it to 15mL of dioxane to prepare a reaction substrate solution. Combine the reaction substrate solution, 0.76mL of anhydrous formic acid and 0.2g of the prepared solution in (1). The Pd / CNT catalyst was mixed and placed in the reaction kettle, the gas in the kettle was replaced with nitrogen five times, the air was exhausted, and the reaction was heated to 120 °C at a stirring rate of 400 r / min for 15 h to obtain the product 2,5-dimethylfuran.
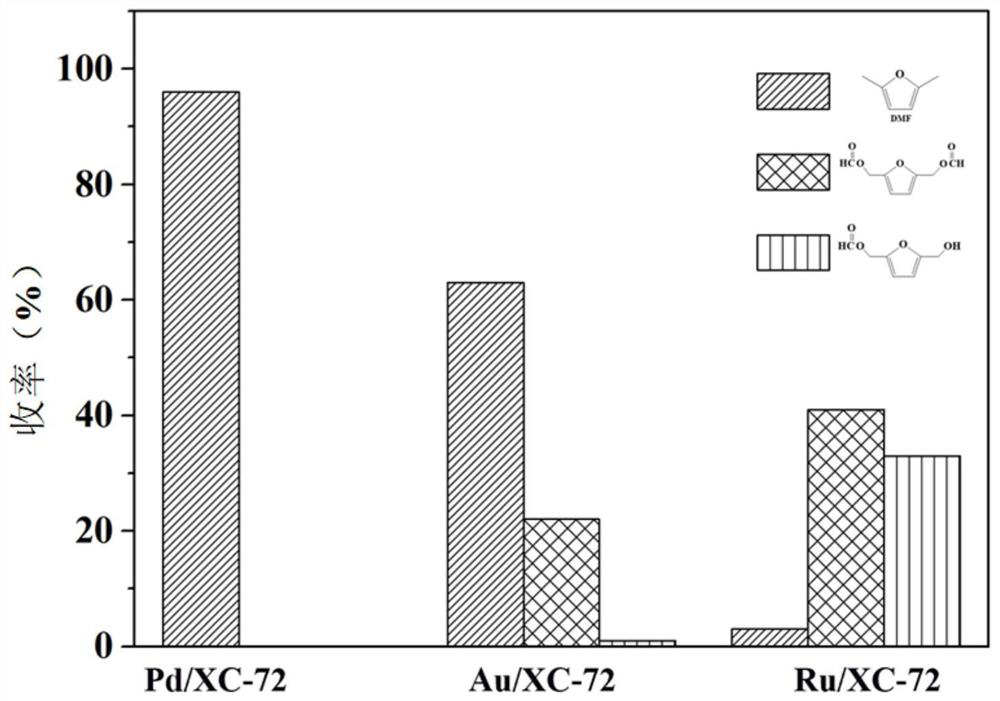
[0079] The reaction results are shown in Table 2. After the reaction for 15 hours, the conversion rate of BHMF reached more than 99%, and the yield of DMF was 73%, w...
Embodiment 3
[0081] (1) Add 0.5g activated carbon, 45mL deionized water, 5mL ethanol to the beaker, add 0.235mmol Na after sonicating for 40min 2 PdCl 4 , and stirred at room temperature for 6h. Then, 5 mL of freshly prepared 0.2 mol / L sodium borohydride solution was added for reduction for 2 h, and the Pd / AC catalyst was obtained by washing.
[0082] (2) Take 0.26g of 2,5-furandimethanol and add it to 15mL of dioxane to prepare a reaction substrate solution. Combine the reaction substrate solution, 0.76mL of anhydrous formic acid and 0.2g of the prepared solution in (1). The Pd / AC catalyst was mixed and placed in the reaction kettle, the gas in the kettle was replaced with nitrogen five times, the air was exhausted, and the reaction was heated to 120 °C at a stirring rate of 400 r / min for 15 h to obtain the product 2,5-dimethylfuran.
[0083] The reaction results are shown in Table 2. After the reaction for 15h, the conversion rate of BHMF reached more than 99%, and the yield of DMF was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com