Device and method for rapidly constructing quinolone compound library
A technology for quinolones and compound libraries, which is applied in the field of devices for rapid construction of quinolones compound libraries. It can solve the problems of low yield, large pollution of three wastes, and long reaction time, and achieve simple reagent switching, accelerated research progress, and fast and efficient reactions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
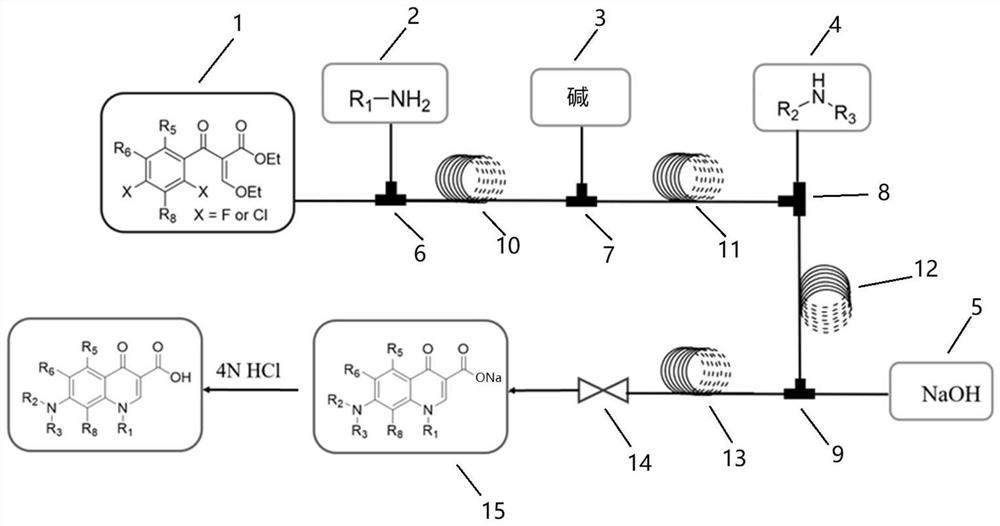
[0062] Because the first feed pump, the second feed pump and the fourth feed pump in the synthesis process need to change different reaction reagents, a multi-channel electromagnetic selector valve is connected to these three pumps respectively, and all pre-prepared The reagents are respectively connected to different positions of the multi-channel electromagnetic selection valve, and the multi-channel electromagnetic selection valve is used to complete the switching of the reagents.
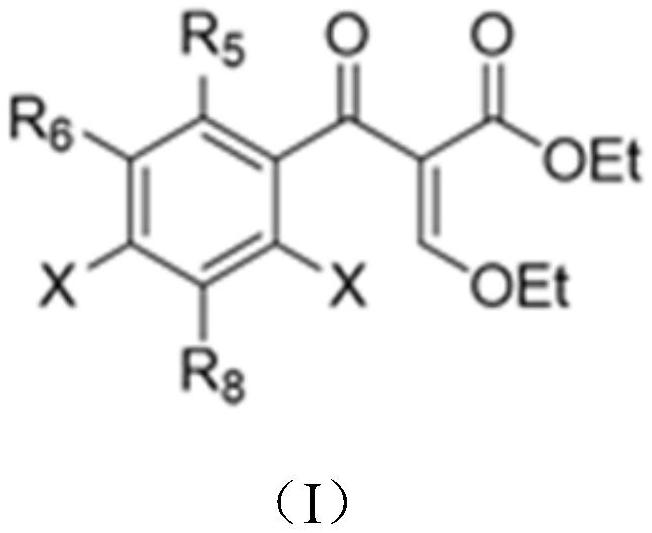
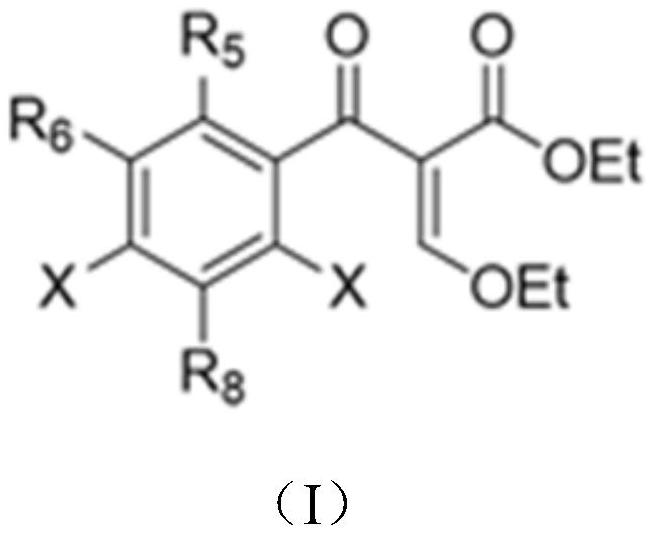
[0063] First, a series of primary amines (cyclopropylamine, ethylamine, 2,4-difluoro) were selected from ethyl 3-ethoxy-2-(2,4,5-trifluorobenzoyl)acrylate Aniline, etc.) and secondary amines (piperazine, N-methylpiperazine, 2-methylpiperazine, 2,6-dimethylpiperazine, 3-aminopyrrolidine, tetrahydropyrrole, piperidine, etc.) to construct A library of quinolones.
[0064] A total of 70 compounds are to be synthesized in this project, and 70 synthesis cycles are required. The operation process of ...
Embodiment 2
[0069] ethyl 3-ethoxy-2-(2,6-dichloro-5-fluoronicotinoyl)acrylate and 3-ethoxy-2-(2,4,5-trifluoro-3-chlorobenzene, respectively formyl) ethyl acrylate as the starting material, a series of primary amines (cyclopropylamine, ethylamine, 2,4-difluoroaniline, etc.) and secondary amines (piperazine, N-methylpiperazine, 2-methyl piperazine, 2,6-dimethylpiperazine, 3-aminopyrrolidine, tetrahydropyrrole, piperidine, etc.) to construct a library of quinolone compounds. According to the method of Example 1, a total of 140 quinolones were synthesized, and the automatically synthesized compounds were as follows:
[0070]
[0071]
Embodiment 3
[0073] Using 3-ethoxy-2-(2,3,4,5-tetrafluorobenzoyl) ethyl acrylate as the starting material and dimethyl sulfoxide as the solvent, S-(+)-2- Aminopropanol is a primary and a series of secondary amines (N-methylpiperazine, piperazine, piperidine, 1-[(3S)-3-pyrrolidine]-piperidine, cis-7-azabicyclo[ 3.3.0] octane, etc.) to construct a library of quinolone compounds. According to the method of Example 1, 9 quinolones including levofloxacin were synthesized, and the automatically synthesized compounds were as follows:
[0074]
[0075] The mass spectrometry characterization data of the compounds in the examples are as follows:
[0076]
[0077]
[0078]
[0079]
[0080]
[0081]
[0082]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com