Preparation method of phenyl dithiourea compound
A technology of phenyldithiourea and compounds, which is applied in the field of COF monomer synthesis, can solve the problems of low yield, poor practicability, and poor universality, and achieve the effects of high yield, expanded structural diversity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
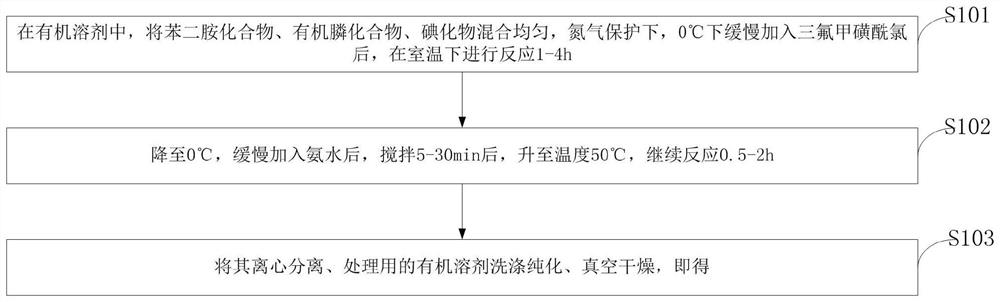
[0039] like figure 1As shown, the present invention provides a preparation method of a phenylbisthiourea compound, comprising the following steps:
[0040] S101, in an organic solvent, the phenylenediamine compound, the organic phosphine compound and the iodide are mixed uniformly, and under nitrogen protection, trifluoromethanesulfonyl chloride is slowly added at 0°C, and the reaction is carried out at room temperature for 1-4 hours.
[0041] S102, drop to 0°C, slowly add ammonia water, stir for 5-30min, raise the temperature to 50°C, and continue the reaction for 0.5-2h.
[0042] S103, centrifuging, washing and purifying with an organic solvent used for processing, and vacuum drying to obtain the obtained product.
[0043] In a preferred embodiment, the iodide is one of sodium iodide, potassium iodide, amine iodide, lithium iodide, cesium iodide and iodine, and the organic phosphine compound is: triphenylphosphine, One of tricyclohexylphosphine, tris(o-methylphenyl)phosphi...
Embodiment 1
[0055] The preparation method embodiment 1 of the 1,4-benzenedithiourea compound of the present invention, the synthetic route is as follows:
[0056]
[0057] Synthesis steps: in 50mL DMF, mix 10mmol of 1,4-phenylenediamine, 25mmol of tricyclohexylphosphine, and 24mmol of potassium iodide. The reaction was carried out for 3h. Drop to 0°C, slowly add ammonia water, stir for 10min, raise the temperature to 50°C, and continue the reaction for 1h. It was centrifuged, purified by washing with THF and water, and dried in vacuo to obtain 1.94 g of a white solid product with a yield of 86%.
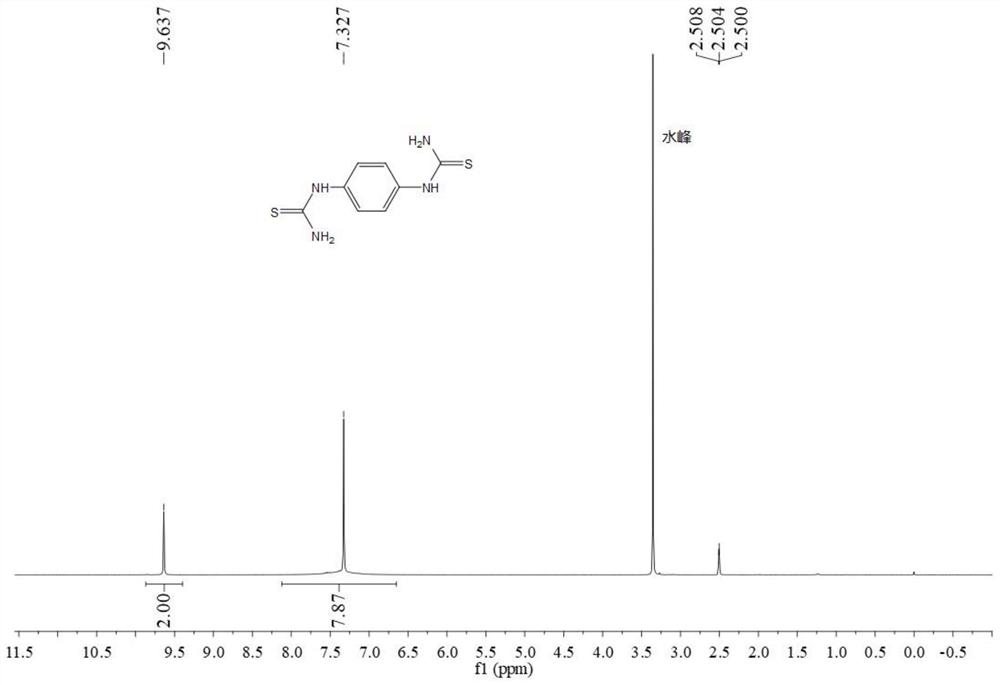
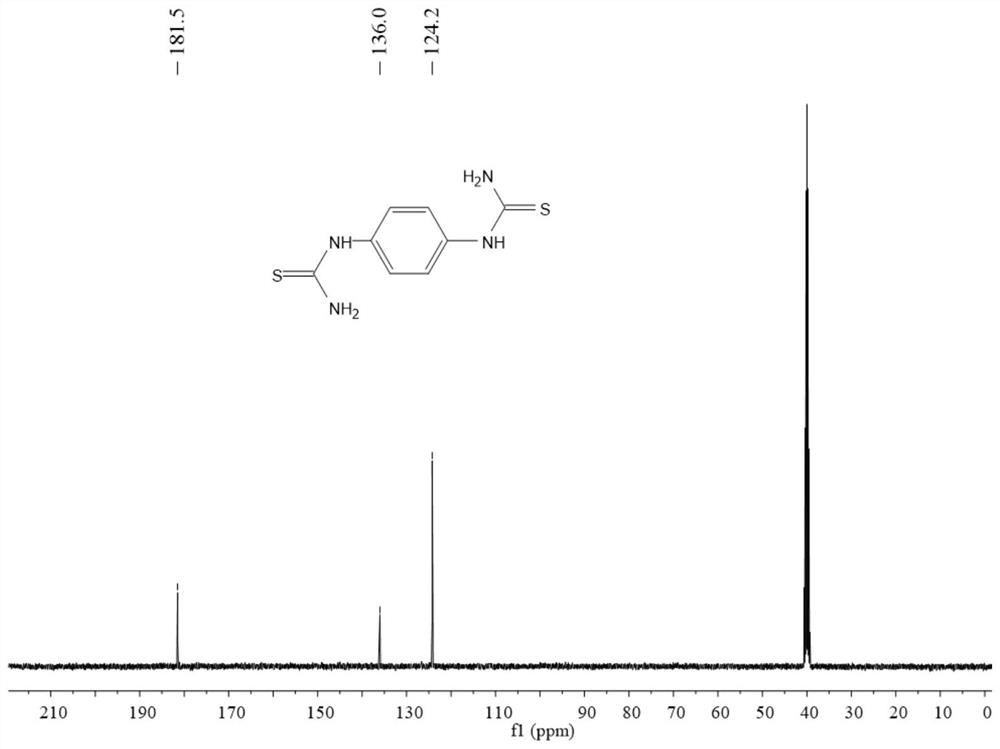
[0058] The H NMR and C spectra of the product are as figure 2 and image 3 As shown, the characterization data is: 1 H NMR (400MHz, deuterated DMSO) δ 9.61 (s, 2H), 7.30 (s, 8H); 13 C NMR (100 MHz, deuterated DMSO) δ 181.5, 136.0, 124.2.
Embodiment 2
[0060] The difference between this example and Example 1 is that the solvent used in the reaction is acetonitrile, the organic phosphine is triphenylphosphine, and the yield is 56%.
[0061] Other steps in this embodiment are the same as those in Embodiment 1, and are not repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com