Synthesis and application of conjugated imide polymer containing dibenzothiophene sulfone
A technology of conjugated imide and benzothiophene sulfone, which is applied in the direction of organic compound/hydride/coordination complex catalyst, chemical/physical process, physical/chemical process catalyst, etc. Inconvenient mechanism research, palladium metal residue and other problems, to achieve the effect of high industrial application value, easy promotion and utilization, and easy acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
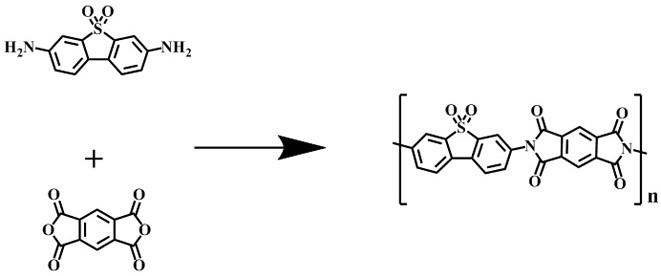
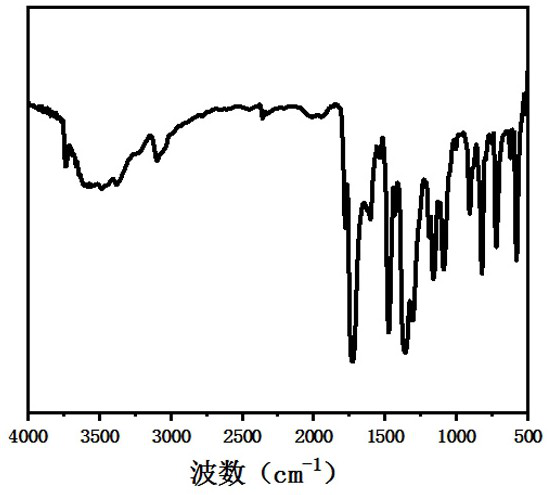
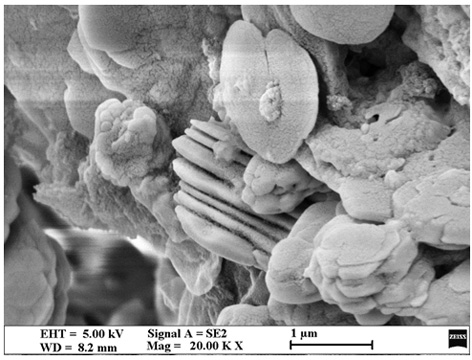
[0020] Combine pyromellitic dianhydride (16.5 mg, 0.075 mmol) and 3,7-diaminodibenzo[b,d]thiophene 5,5-dioxide (18.5 mg, 0.075 mmol) and 1.05 mL isoquinoline / N-methylpyrrolidone (NMP) / mesitylene (Mes) mixed system (1:10:10, v / v / v) placed in a Pyrex tube (volume about 5mL, body length 20 cm, neck length 1 cm) , after ultrasonic treatment for 10 minutes, the Pyrex tube was quickly frozen in a liquid nitrogen bath and then vacuumed, thawed and then frozen and vacuumed, and the thawing-freezing-vacuuming was repeated three times until the internal pressure of the container was 0 mbar, and flame sealing was performed. After warming up to room temperature, the closed Pyrex tube was placed in an oven at 200 °C for 5 days, resulting in a yellow solid. The precipitate was collected by suction filtration, washed three times with tetrahydrofuran, washed three times with acetone, and vacuum-dried at 80 °C overnight to obtain a mixture containing dihydrofuran. Conjugated imide polymers (S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com