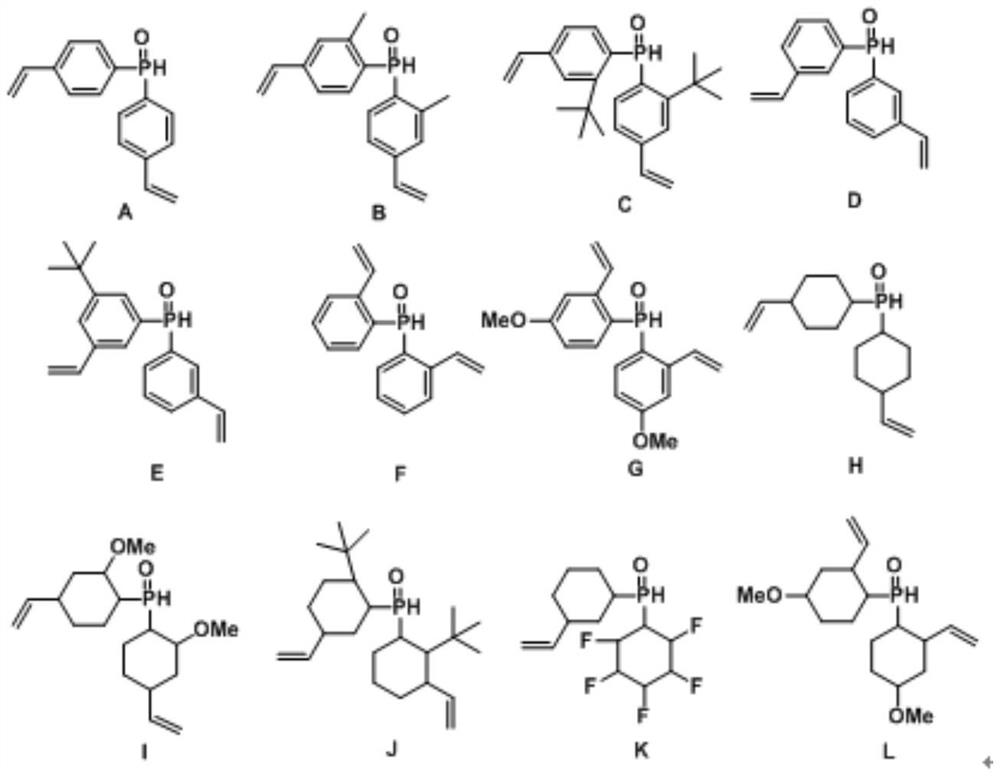
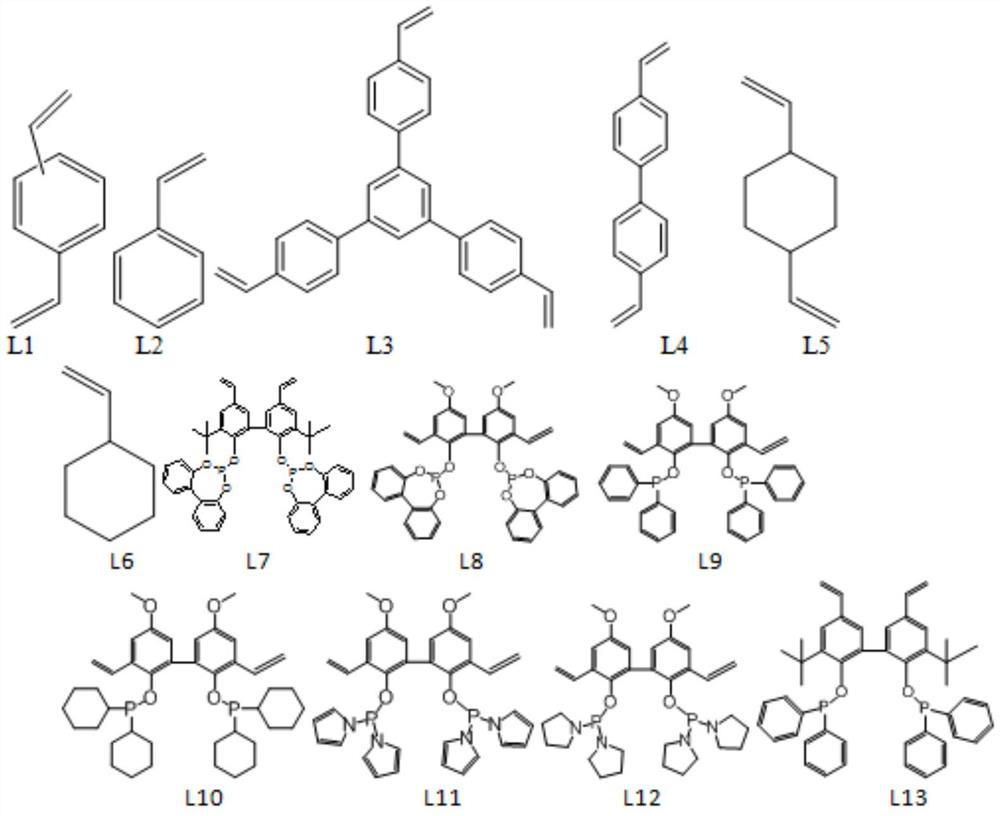
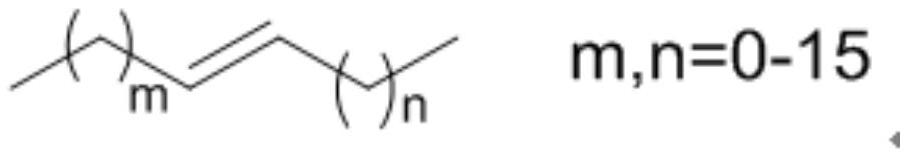Method for internal olefin hydroformylation reaction by using phosphine oxide polymer supported catalyst
A supported catalyst and olefin hydroformyl technology, which is applied in the direction of organic compound/hydride/coordination complex catalyst, catalytic reaction, carbon monoxide reaction preparation, etc., can solve the problems of low activity and poor conversion of internal olefins, and achieve High catalytic activity, stable air and moisture, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The specific preparation steps of monomer B: 5°C, under the protection of Ar gas, 12.8g of 1-bromo-2-methyl-4-vinylbenzene (CAS No.: 90560-53-5) was dissolved in 50ml of tetrahydrofuran, stirred evenly and waited for use. 2.0g of Mg chips were put into the flask, the temperature of the flask was raised to 65°C, and 5 ml of the mixed solution of 1-bromo-2-methyl-4-vinylbenzene and tetrahydrofuran was added dropwise. into dark green, vigorously boiling) continue to add the remaining mixed solution dropwise, maintaining the dropwise temperature at 60°C. After the dropwise addition, the solution was incubated for 1 hour to obtain the Grignard reagent solution. After cooling to 0 degrees Celsius, a mixed solution of 4.5 g of diethyl phosphite and 50 ml of 2-methyltetrahydrofuran was added, and the reaction was continued for 1 h after the dropwise addition was completed. Add 10 ml of saturated ammonium chloride solution to annihilate the reaction, the mixture is divided int...
Embodiment 2
[0049] In Example 2, except that 50.0 g of the second component, divinylbenzene, was not added, the rest of the synthesis process and conditions were the same as those in Example 1.
Embodiment 3
[0051] In Example 3, except that 50.0 g of the second component tristyrylbenzene (ligand L3 in claim 3) was used instead of divinylbenzene (ligand L1), the rest of the synthesis process and conditions were the same as those in Example 1 same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com