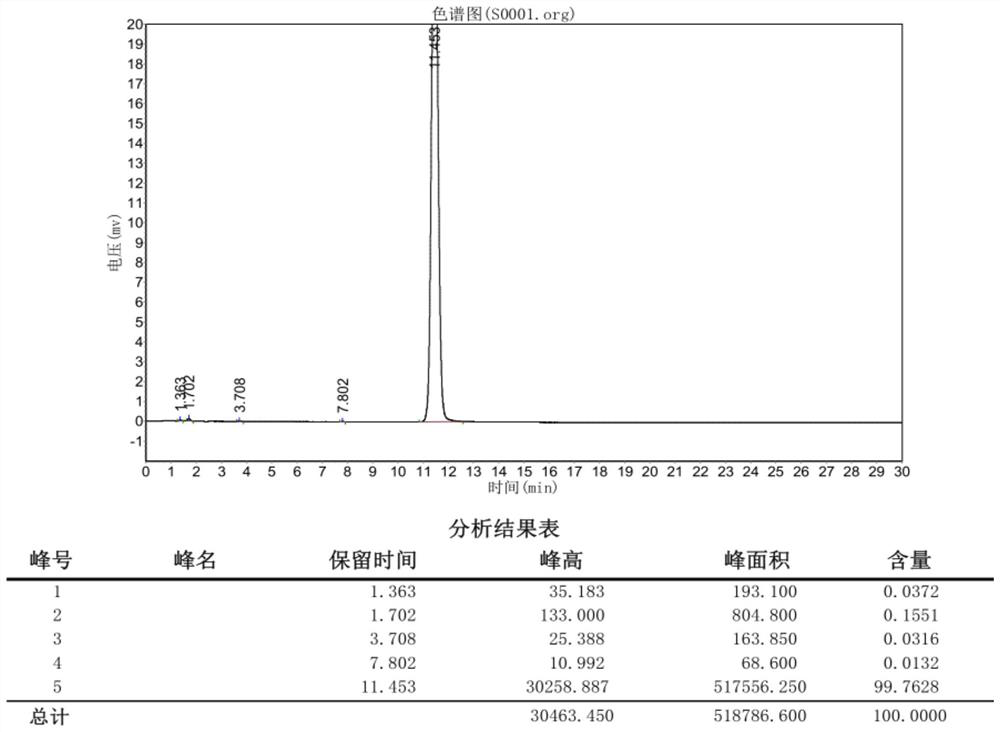
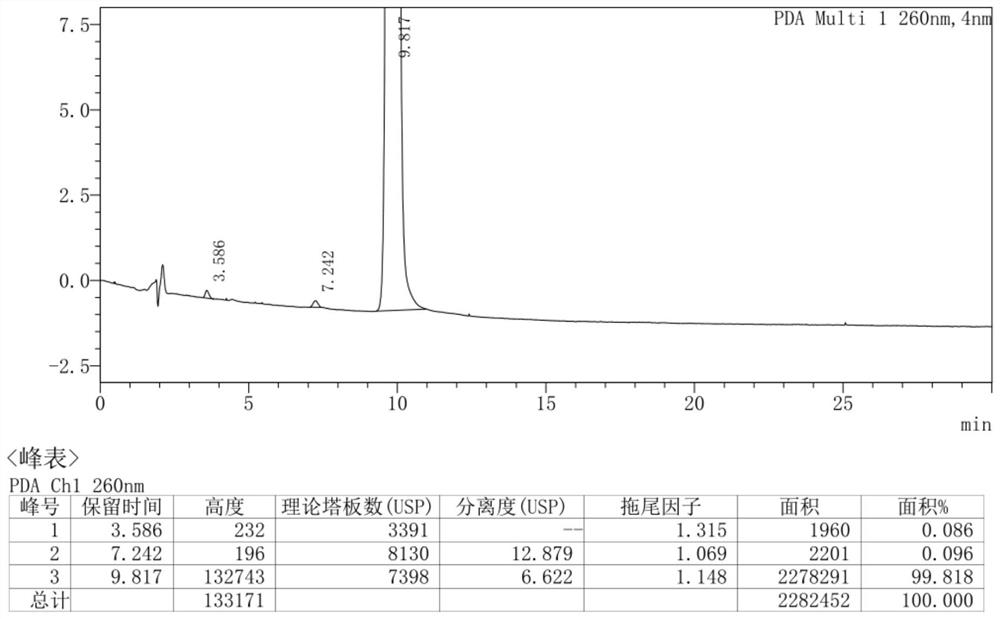
Preparation method of (S)-1, 2, 3, 4-tetrahydro-1-naphthoic acid
A technology of naphthoic acid and hydrochloric acid, applied in the field of medicine and chemical industry, can solve problems affecting product quality, safety hazards, quality defects, etc., achieve the effect of improving chiral purity and chemical purity, eliminating safety hazards, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0045]12.9 kg of 1,2,3,4-tetrahydro-1-naphthoic acid, 23.8 kg of quinine, and 275 liters of 90% ethanol aqueous solution were added to the reaction vessel, and stirred evenly to obtain a mixed liquid, and the temperature was slowly raised to The mixture was completely dissolved, kept for 10 minutes, slowly cooled to 0~5℃, crystallized for 16 hours, a solid was precipitated, filtered and dried to obtain (S)-1,2,3,4-tetrahydro-1 -12.5 kg of crude naphthoic acid quinine salt.
[0046] In the reaction vessel, add 12.5 kg of the crude product of (S)-1,2,3,4-tetrahydro-1-naphthoic acid quinine salt obtained in the previous step, 175 liters of anhydrous ethanol, stir evenly, heat up to reflux, cool down To 0~5 ℃, stand for crystallization for 16 hours, there is solid precipitation, filter, oven dry to obtain 9.18 kg of fine products of (S)-1,2,3,4-tetrahydro-1-naphthoic acid quinine salt .
[0047] After the two steps of salification and recrystallization are combined, the molar yi...
Embodiment 1B
[0049] Add 12.9 kg of 1,2,3,4-tetrahydro-1-naphthoic acid, 23.8 kg of quinine, and 325 liters of anhydrous ethanol to the reaction vessel, stir well to obtain a mixed liquid, and slowly heat up until all the mixed substances are Dissolve, keep warm for 10 minutes, slowly cool down to 0~5℃, crystallize for 16 hours, there is solid precipitation, filter and dry to obtain (S)-1,2,3,4-tetrahydro-1-naphthoic acid· Crude quinine salt 15.2 kg.
[0050] Add 15.2 kg of the crude product of (S)-1,2,3,4-tetrahydro-1-naphthoic acid quinine salt obtained in the previous step, and 115 liters of 85% ethanol aqueous solution obtained in the previous step into the reaction vessel, stir evenly, slowly Slowly heat up until the mixture is completely dissolved, cool down to 0~5°C, stand for crystallization for 16 hours, a solid is precipitated, filter, and dry to obtain (S)-1,2,3,4-tetrahydro-1- Naphthoic acid quinine salt boutique 8.98 kg. After the two steps of salification and recrystallizati...
Embodiment 2A
[0052] 8 kg of (S)-1,2,3,4-tetrahydro-1-naphthoic acid quinine salt obtained in Example 1 was added to 25 liters of water and 40 liters of ethyl acetate, stirred, and 1 mol / l of ethyl acetate was added dropwise. Hydrochloric acid until the pH value reaches about 2.0, the layers are separated, and the organic layer is washed with 20 liters of 0.5 mol / l hydrochloric acid again. The aqueous layer was discarded, and the organic layer was used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com