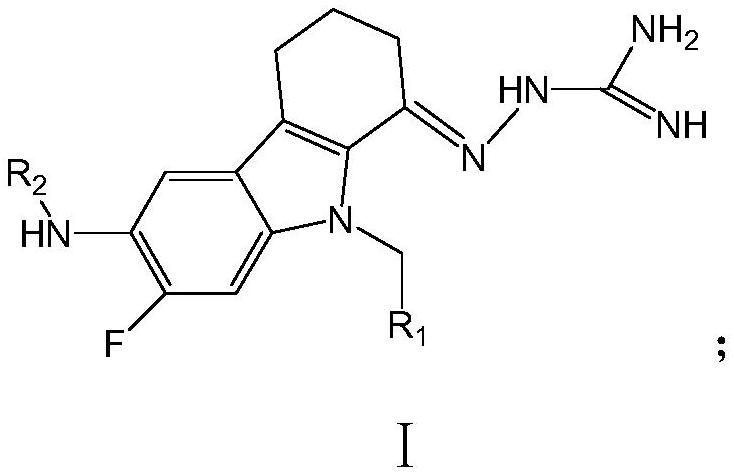
Tetrahydrocarbazole derivative as well as preparation method and application thereof
A technology for tetrahydrocarbazole and derivatives is applied in the field of tetrahydrocarbazole derivatives and their preparation, which can solve the problems of unsatisfactory anti-tuberculosis effect and drug resistance, and achieve excellent anti-tuberculosis effects, The effect of less side reactions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
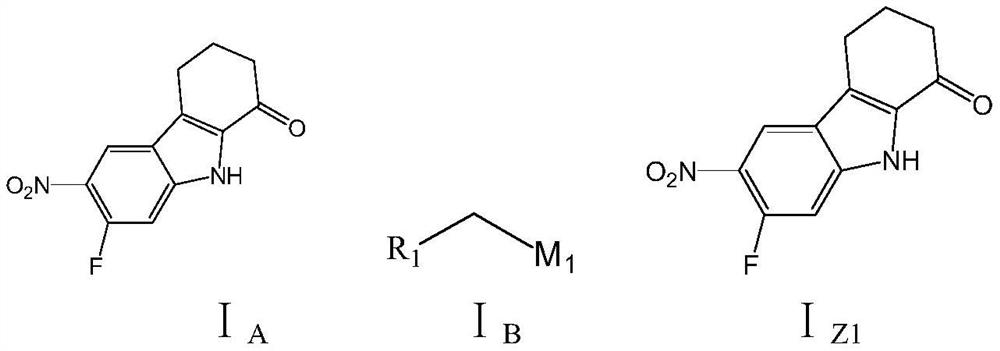
[0039] On the other hand, the examples of the present application provide the preparation methods of the tetrahydrocarbazole derivatives in the examples of the above-mentioned application. The preparation method of the tetrahydrocarbazole derivative of the embodiment of the present application comprises the following steps:
[0040] S01: the following structural formula I A The reactant A shown is with the following structural formula I B The shown reactant B and the base catalyst carry out the first substitution reaction in the first reaction solvent to generate the following structural formula I Z1 the first intermediate product shown;
[0041]
[0042] S02: the first intermediate product is reversely carried out redox reaction with reducing agent and acid additive in the second reaction solvent to generate the following structural formula I Z2 the second intermediate product shown;
[0043]
[0044] S03: combine the second intermediate product with the following s...
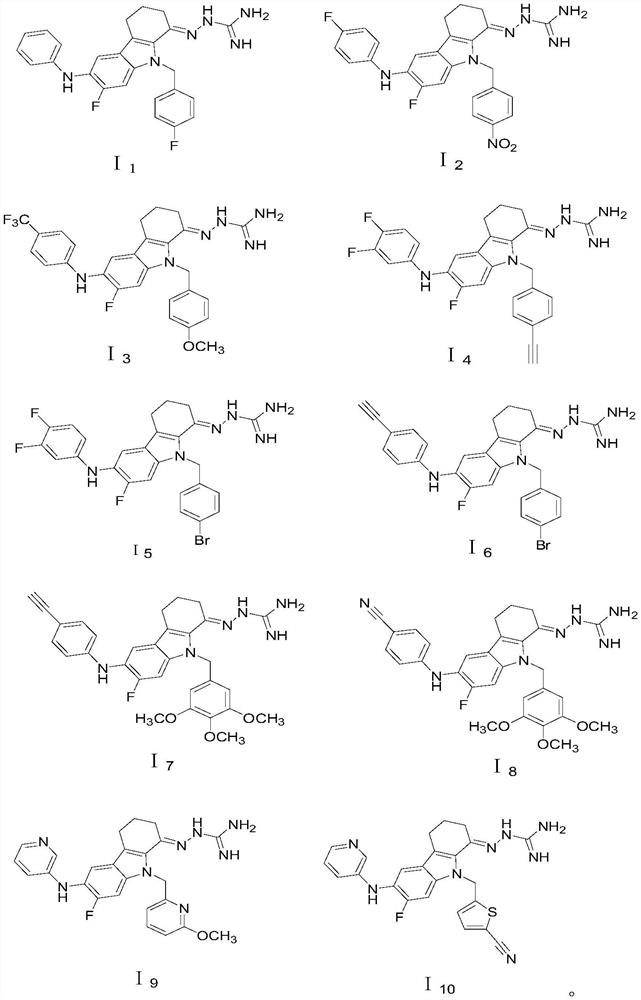
Embodiment 1
[0094] This embodiment provides a 2,3,4,9-tetrahydrocarbazole derivative and a preparation method thereof. The structural formula of the 2,3,4,9-tetrahydrocarbazole derivative is the above formula I 1 shown.
[0095] Formula I above 1 The preparation method of the 2,3,4,9-tetrahydrocarbazole derivatives shown comprises the following steps:
[0096] S1: Synthesis of Reactants A3 and A above:
[0097] Synthesis of reactant A3: According to the synthetic route shown in the above reaction formula (1), in a 1000 mL round-bottomed flask, 30 g of 4-nitro-5-fluorophenylhydrazine, 21 g of 1,2-cyclohexanedi 700 mL of ketone and 1M hydrochloric acid solution, then refluxed and stirred for 12 hours; cooled and filtered to obtain crude product 1, which was directly carried out to the next step without purification.
[0098] The synthesis of reactant A: according to the synthetic route shown in the above reaction formula (1), to the synthetic reactant A3 crude product was added concentr...
Embodiment 2
[0109] This embodiment provides a 2,3,4,9-tetrahydrocarbazole derivative and a preparation method thereof. The structural formula of the 2,3,4,9-tetrahydrocarbazole derivative is the above formula I 2 shown.
[0110] Formula I above 2 The preparation method of the 2,3,4,9-tetrahydrocarbazole derivatives shown comprises the following steps:
[0111] S1: The synthesis of reactant A3 and reactant A are respectively synthesized with reference to step S1 in Example 1:
[0112] S2: Synthesize the above structural formula I with reference to step S2 in Example 1 Z1 The first intermediate product shown. The difference is that the reactant p-fluorobenzyl bromide is replaced with p-nitrobenzyl bromide.
[0113] S3: Synthesize the above structural formula I with reference to step S3 in Example 1 Z3 The second intermediate product shown.
[0114] S4: Synthesize the above structural formula I with reference to step S4 in Example 1 Z3 The third intermediate product shown. The diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com