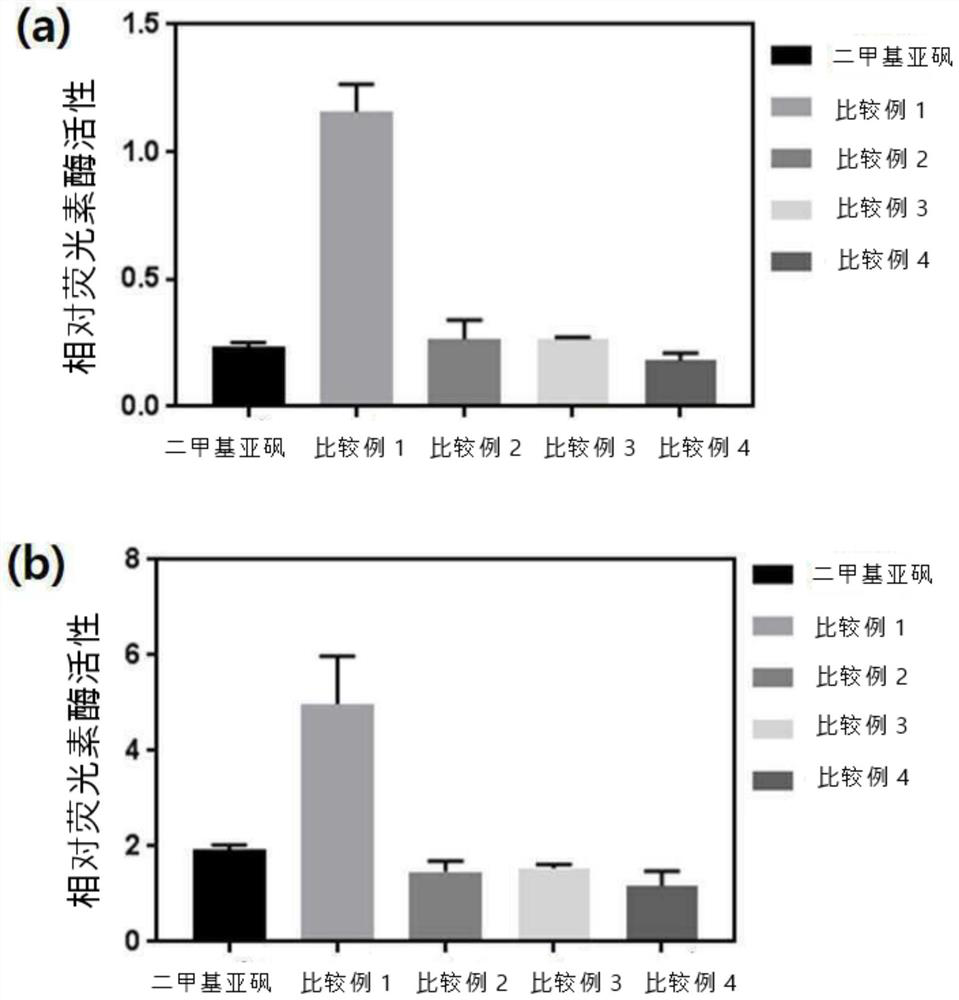
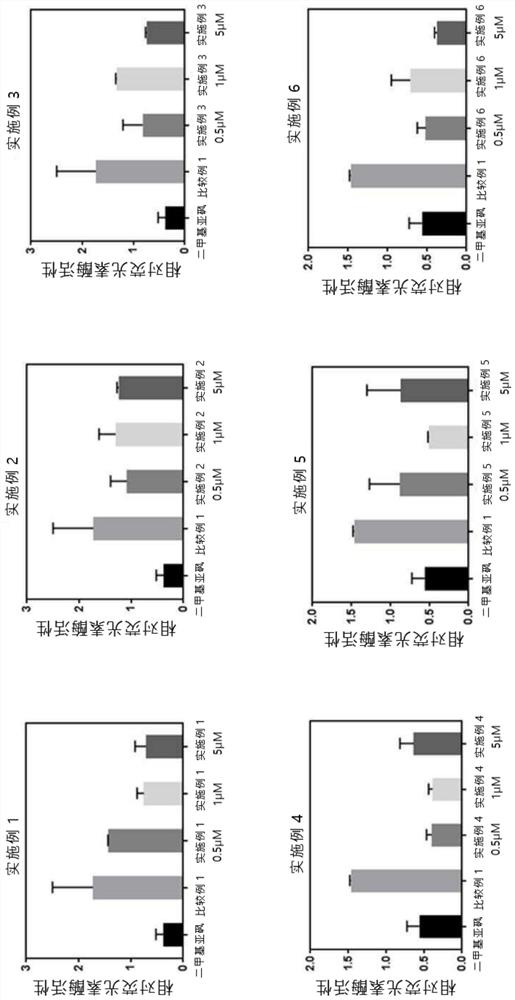
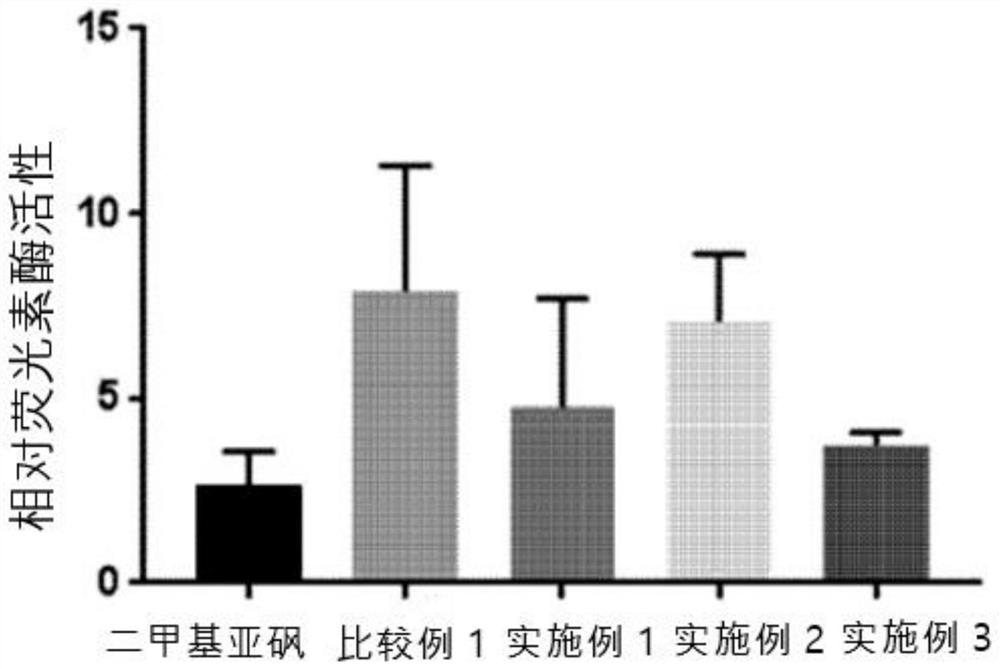
Compound for inducing expression of anti-aging gene KLOTHO and application thereof
A compound and alkyl technology, applied in the field of expressed compounds, can solve problems such as the reduction of klothomRNA expression, achieve excellent effects, prevent skin aging, and inhibit cell aging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] The present invention provides the preparation method of the compound represented by chemical formula 1A, it is as following reaction formula 1, comprises the following steps:
[0142] Step 1, after dissolving compound 2 in an organic solvent, adding compound 3, reacting at a temperature of 10°C to 50°C for 12 hours to 20 hours to obtain compound 4; and
[0143] Step 2, add dropwise the organic solvent in which compound 4 obtained in the above step 1 is dissolved in the organic solvent in which potassium superoxide is dissolved, and react at a temperature of 15°C to 30°C for 10 hours to 16 hours to obtain compound 1A .
[0144] Reaction 1:
[0145]
[0146] In the above reaction formula 1,
[0147] R 1 , R 2 , R 3 , R 4 , R 5 , R 6 and R 7 As defined in the chemical formula 1 of claim 1,
[0148] The above compound 1A is contained in the chemical formula 1 of claim 1 .
[0149] In the preparation method of the present invention, as examples of the above-ment...
Embodiment 1
[0233] Example 1: N-(Benzo[d]oxazol-2-yl)-2-chloro-4-nitrobenzamide (N-(Benzo[d]oxazol-2-yl)-2-chloro- 4-nitrobenzamide, FCCS-17064) preparation
[0234]
[0235] Step 1: Dimethyl(2-chloro-4-nitrobenzoyl)iminodithiocarbonate (Dimethyl(2- Preparation of chloro-4-nitrobenzoyl)carbonimidodithioate, 17064-2-1)
[0236] 2-Chloro-4-nitrobenzamide (2-Chloro-4-nitrobenzamide) (500mg, 2.49mmol), carbon disulfide (Carbon disulfide, CS 2 ) (759mg, 9.97mmol), iodomethane (1.13g, 7.97mmol) were dissolved in dimethylformamide (N,N-dimethylformamide) (7mL), and 60% sodium hydride (200mg, 4.98mmol) was added, and Stir at room temperature for 5 hours.
[0237] Ice-cold water was slowly added to the reaction mixture and ethylacetate was extracted. The organic layer was washed with brine, and Na 2 SO 4 After drying, the solvent was removed under reduced pressure. Purification of the reaction mixture by Silica-gel column chromatrography (10% Ethyl acetate / n-hexane) afforded dimethyl(2-...
Embodiment 2
[0243] Example 2: 8-Methyl-2-[N-(3,4-dichlorophenyl)]aminobenzoxazole (8-Methyl-2-[N-(3,4-dichlorophenyl)]aminobenzoxazole, FCCS-17065) Preparation
[0244]
[0245] Step 1: 1-(3,4-dichlorophenyl)-3-(2-hydroxy-5-methylphenyl)thiourea (1-(3,4- Preparation of Dichlorophenyl)-3-(2-hydroxy-5-methylphenyl)thiourea, 17065-2-1)
[0246] After dissolving 2-amino-p-cresol (2-Amino-p-cresol) (300mg, 2.44mmol) in methanol (methanol) (12mL), add 3,4-dichlorophenylisothiocyanate ( 3,4-dichlolrophenyl isothiocyanate) (497 mg, 2.44 mmol) and stirred at room temperature for 18 hours. After confirming the completion of the reaction by thin layer chromatography (thin layer chromatography, TLC), it was cooled in a refrigerator (0° C. to 4° C.). The precipitated solid was filtered to obtain 1-(3,4-dichlorophenyl)-3-(2-hydroxy-5-methylphenyl)thiourea as a white solid (346 mg), which was used directly in next step.
[0247] 1 H NMR (400MHz, acetone-d 6 ); J = 8.4Hz) 2.24(s, 3H).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com