Preparation method of fusion enzyme and fusion enzyme
A technology of fusion enzyme and crude enzyme liquid, applied in the field of fusion enzymes, can solve the problems of lack of fusion enzyme preparation and purification scheme, low reaction catalytic efficiency, low catalytic activity, etc., and achieves good stability, improved enzyme catalytic activity, and efficient catalysis. effect of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
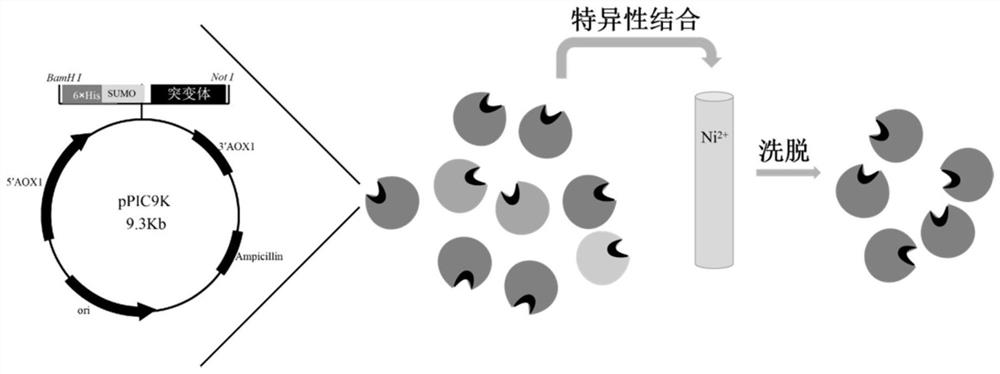
[0051] This embodiment relates to a preparation method of a fusion enzyme, and an exemplary preparation schematic diagram thereof is as follows figure 1 Shown; This preparation method mainly comprises following three steps:
[0052] 1. Construct fusion enzyme plasmid;
[0053] 2. Extract the fusion enzyme plasmid and transfer it into Pichia pastoris for fermentation and expression;
[0054] 3. Utilize Pichia pastoris to prepare crude enzyme solution, and carry out separation and purification of fusion enzyme.
[0055] The main body of the fusion enzyme is UPO mutants JaWa, SoLo, WamPa and the C-terminal 6×His-SUMO tag. The specific affinity between histidine and metal ions makes the separation and purification process of the enzyme simple and efficient.
[0056] Based on the overall setting principle of the preparation and purification method of the fusion enzyme mentioned above in this embodiment, the following specific steps can be referred to for the preparation.
[00...
Embodiment 1
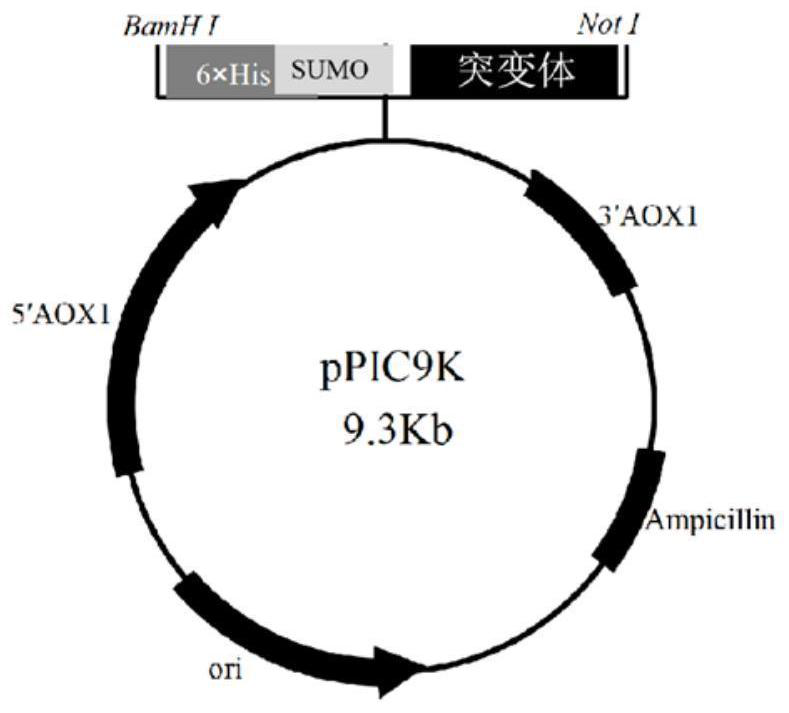
[0120] This example relates to the preparation of 6×His-SUMO-JaWa, 6×His-SUMO-SoLo, 6×His-SUMO-WamPa fusion enzyme plasmids. The principle of plasmid extraction from Escherichia coli, and subsequent linearization and purification is as follows: figure 1 shown.
[0121] (1) Construct mutants JaWa, SoLo, and WamPa of non-specific peroxidase (AaeUPO) from Agrocybe aegerita, and fuse 6×His-SUMO gene at their C-terminus. Construct the fusion enzyme gene sequence, and then use restriction endonucleases BamHI and NotⅠ to connect the fusion enzyme gene sequence to plasmid pPIC9K to construct recombinant plasmids pPIC9k-6×His-SUMO-JaWa, pPIC9k-6×His-SUMO-SoLo , pPIC9k-6 x His-SUMO-WamPa.
[0122] The schematic diagram of the constructed plasmid is shown in figure 2 shown. The following table 1 lists the protein sequence of the fusion enzyme
[0123] Table 1. Protein sequences of fusion enzymes
[0124]
[0125] (2) Transfer the above constructed plasmid into the competent...
Embodiment 2
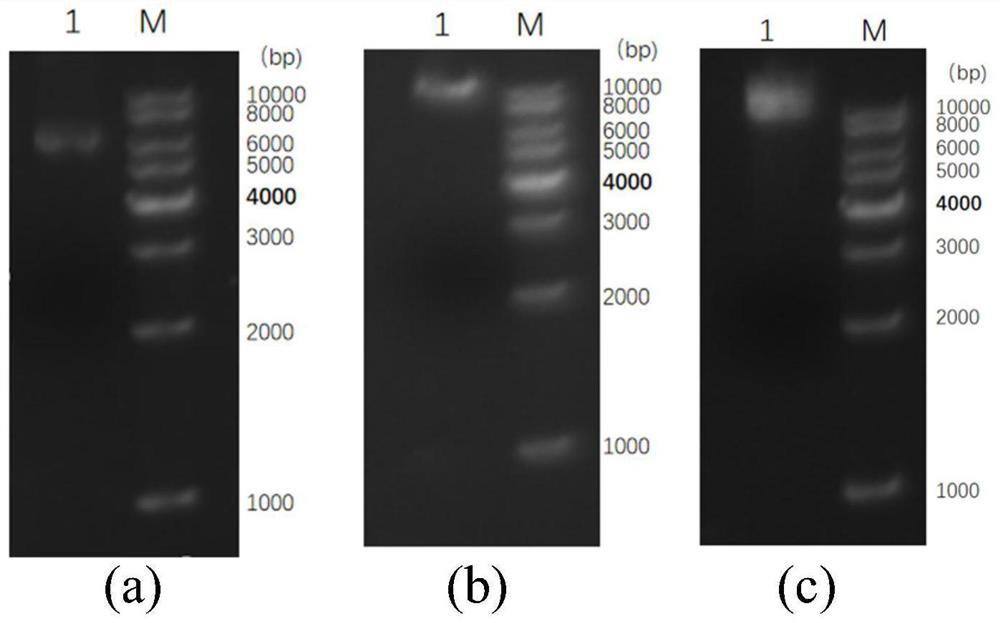
[0130] This example involves the electroporation of fusion plasmids into competent Pichia cells and obtaining positive bacteria. The colony picture of positive bacteria on the MD plate is as follows Figure 4 shown. The plasmid extraction map and PCR verification electrophoresis map of positive bacteria are as follows: Figure 5 In a, b shown.
[0131] (1) Firstly, Pichia pastoris was activated with YPD medium, and the culture medium was inoculated from Glycerol bacteria at an inoculum size of 1%.
[0132] (2) Continue to inoculate into two new YPDs with 1% inoculum size, expand and cultivate Pichia pastoris, and wait until OD 600 To 1.2, the bacteria solution was centrifuged. The centrifugation conditions were 5000 rpm, 5 min, 4°C. After centrifugation, the supernatant was discarded, leaving the pellet. Use 10-15 mL of pre-cooled sterile water to mix the precipitate by pipetting, continue to centrifuge, and repeat the operation 2-3 times. Finally, it was resuspended wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com