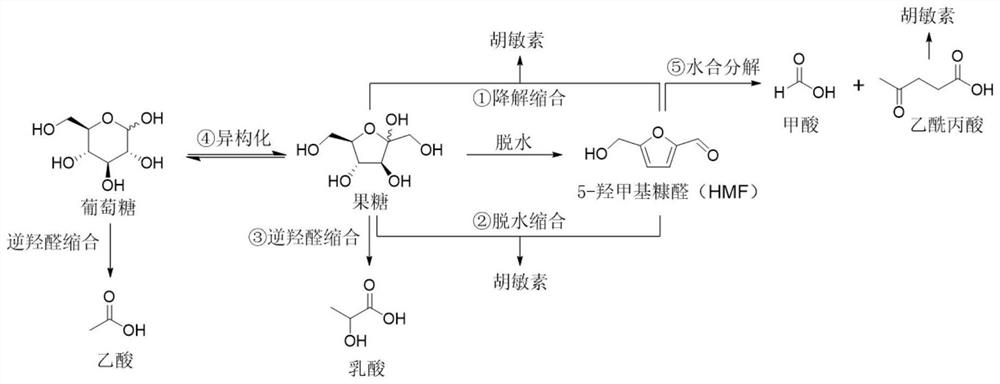
Synthetic method of 5-hydroxymethylfurfural promoted by surfactant
A surfactant, hydroxymethyl furfural technology, applied in organic chemistry and other directions, can solve the problems of low utilization rate of raw material carbon, low HMF synthesis efficiency, high synthesis energy consumption, and reduce by-products such as small molecular organic acids and humins. The generation of raw material, high utilization rate of raw material carbon, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Add magnetic stir bar, 500.0mg fructose, 0.95mL 1,4-dioxane, 0.05mL water, 5.0mg cetyltrimethylammonium bromide (CTAB) and 2.0mg H 2 SO 4 Catalyst, nitrogen purge for 3 minutes to remove the air in the thick-walled glass pressure tube. The temperature of the oil bath was raised to 140°C, and after the temperature became constant, a pressure-resistant tube was placed, and the reaction was stirred and heated for 15 minutes under airtight conditions. After the reaction stopped, the reaction system was naturally cooled to room temperature, and the solution was taken out for HPLC analysis. The yield of HMF was 63.3%.
Embodiment 2-15
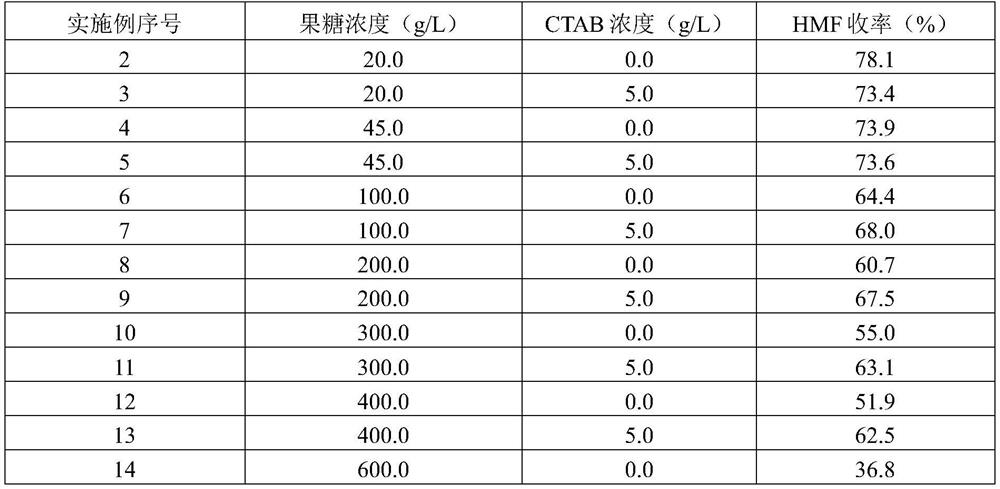
[0015] According to the method of Example 1, under the condition of adding CTAB (5.0g / L) or not adding CTAB, using different concentrations of fructose as the substrate for reaction, the reaction conditions and results are listed in Table 1.
[0016] Table 1
[0017]
[0018]
Embodiment 16-18
[0020] According to the method of Example 1, different volume ratios of 1,4-dioxane and water were used as mixed solvents for the reaction. The reaction conditions and results are listed in Table 2.
[0021] Table 2
[0022] Example serial number V 1,4-二氧六环 :V 水
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com