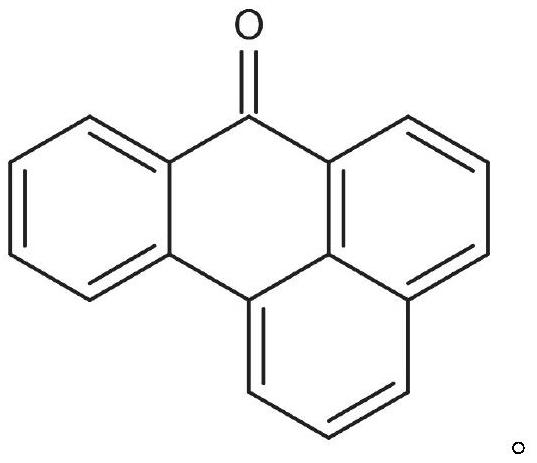
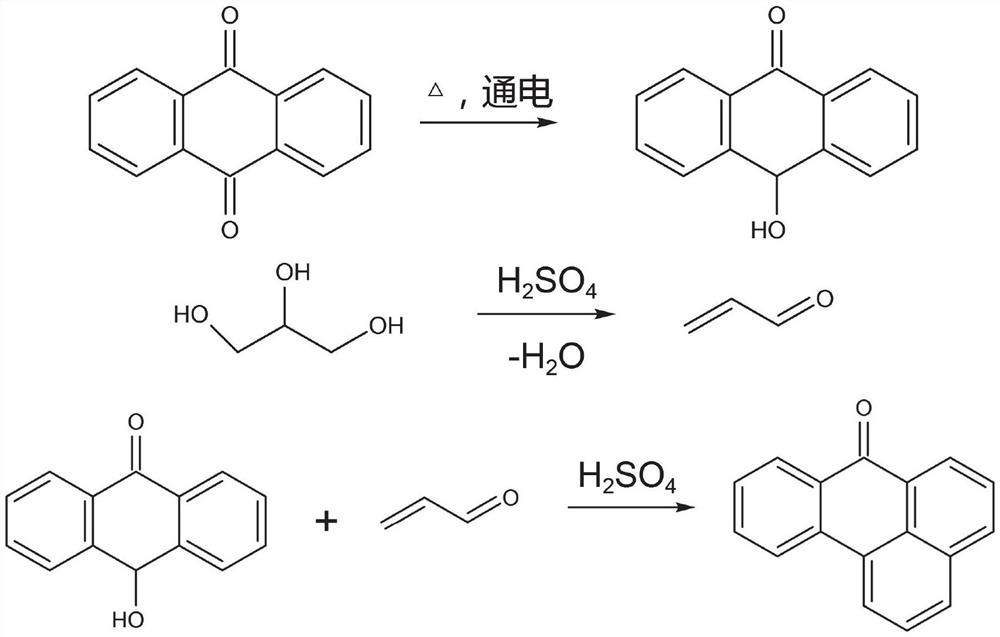
Electrochemical preparation method of benzanthrone
A benzranthrone and electrochemical technology, applied in the field of electrochemical organic synthesis, can solve problems such as environmental pollution, complex synthesis process, etc., and achieve the effects of simple process, mild reaction conditions and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 0.048mol of anthraquinone and 50g of 85% sulfuric acid solution into the electrolytic cell with carbon electrodes as cathode and anode, respectively, under nitrogen protection, stir and heat the solution to 100°C at a heating rate of 1°C / min, and energize. , adjust the current to 3A, and dropwise add 0.1mol glycerol for 2h; when the passing electricity reaches 10560 coulombs, stop energizing; dilute the concentration of sulfuric acid solution to 60%, filter and wash the filter cake to pH 6 After ~7, it was dried in a constant temperature oven at 105 °C for 12 h to obtain benzrathranthrone.
Embodiment 2
[0027] Add 0.048mol of anthraquinone and 50g of 85% sulfuric acid solution into the electrolytic cell with platinum electrode as cathode and glass electrode as anode, under nitrogen protection, stir and heat the solution to 80°C at a heating rate of 2°C / min ; Power on, adjust the current to 10A, and add 0.048mol glycerol dropwise at the same time, the dropping time is 1h; when the passing electricity reaches 10560 coulombs, stop the power; Dilute the concentration of the sulfuric acid solution to 60%, filter and wash the filter cake to pH After being 6 to 7, it was placed in a constant temperature oven at 105° C. to dry for 6 hours to obtain benzrathrone.
Embodiment 3
[0029] Add 0.048mol of anthraquinone and 50g of 85% sulfuric acid solution into the electrolytic cell with platinum electrodes as cathode and anode respectively, under nitrogen protection, stir and heat the solution to 120°C at a heating rate of 2°C / min; , adjust the current to 3A, and add 0.48mol glycerol dropwise at the same time for 4h; when the passing electricity reaches 10560 coulombs, stop energizing; dilute the concentration of the sulfuric acid solution to 60%, filter and wash the filter cake to pH 6 After ~7, it was dried in a constant temperature oven at 105°C for 12 h to obtain benzrathrone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com