Cell for expressing phosphotransferase and non-ribosomal peptide synthetase and application thereof
A technology of phosphotransferase and peptide synthetase, applied in the direction of transferase, enzyme, bacteria, etc., can solve the problems of decarboxylated carnosine with many by-products and large pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
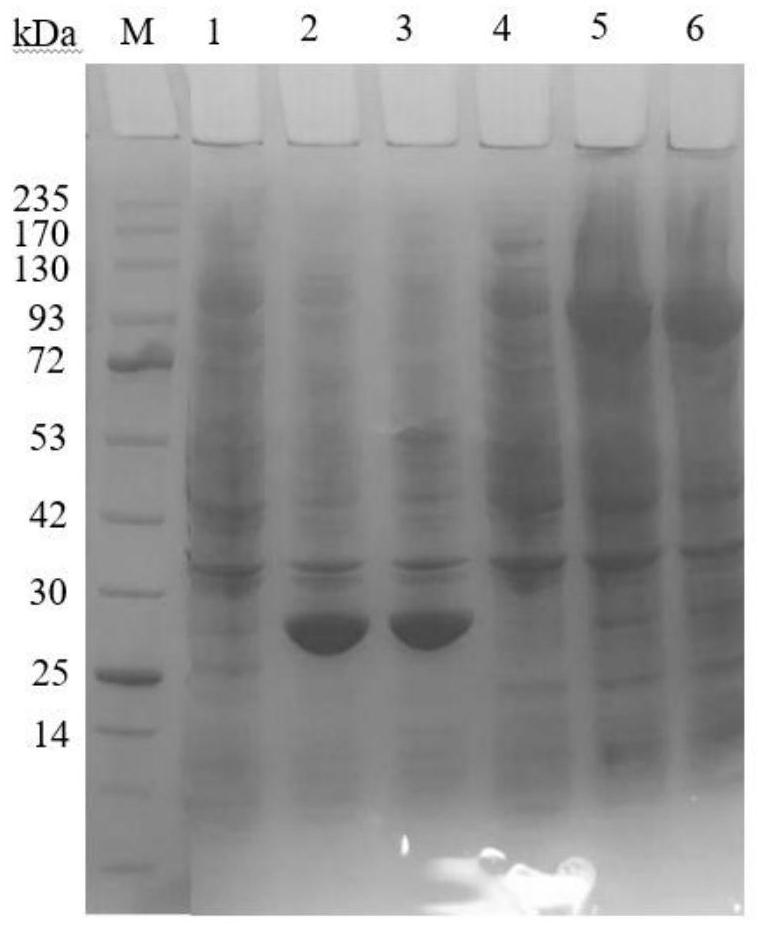
Image
Examples
Embodiment 1
[0050] Example 1: Construction of recombinant expression plasmids
[0051] 1. Primers
[0052] The sfp, ebony gene, p15A replicon and pET28a, pACYC, pTrc99A, pTrc-p15A vectors were amplified in vitro as follows:
[0053] (1) construct the pACYC-sfp plasmid; the PCR primer sequences used in this embodiment are listed in Table 5, and the Bsusfp gene is amplified in vitro (the nucleic acid sequence is as shown in SEQ ID NO.1, derived from the Bacillus subtilis genome, and the primer pair Bsusfp-F, Bsusfp-R), linearize the pACYC plasmid (pACYCDuet-1 plasmid, the primer pair is pACYC-F, pACYC-R) by inverse PCR at the MCS site. The PCR-amplified products of the above plasmids were digested with endonuclease Dpn I at 37°C for 1 h, the template DNA was removed, purified with a purification kit, and then purified using the ClonExpress II One Step Cloning Kit (Novizan Biotechnology Co., Ltd., Cat. No.: C112-01 ) One-step cloning of Bsusfp gene and linearized pACYC fragment (one-step c...
Embodiment 2
[0073] Embodiment 2: Construction of recombinant genetically engineered bacteria
[0074] The transformant that embodiment 1 obtains is carried out as follows:
[0075] 1. Sequencing verification: use a sterilized pipette tip to select 5 transformants on the plate for colony PCR (the primers used are shown in Table 6). The correct bands were inoculated in LB liquid medium containing the corresponding resistance (see Table 7), cultured at 37°C with shaking at 200r / min for 12 hours, sequenced and preserved. The constructed strains are shown in Table 7.
[0076] Transformant required colony PCR primers in table 6 embodiment 1
[0077]
[0078] The bacterial strain constructed in the embodiment 2 of table 7
[0079]
[0080]
[0081] 2. Induced expression of recombinant plasmids: under sterile conditions, take 1 mL of seed liquid from the LB liquid medium in step 1, and transfer to 50 mL of LB medium (containing Kana at a final concentration of 100 μg / mL; containing Kan...
Embodiment 3
[0082] Embodiment 3: Preparation reaction and detection of decarboxylated carnosine
[0083] 1. Centrifuge the thalline containing the induced recombinant genetically engineered bacteria at 12000rpm for 15min, discard the supernatant and collect the thalline; the collected thalline is resuspended using 20mL pH 8, 50mM trisaminomethane (Tris-HCl) buffer , 8000rpm, high-speed centrifugation for 15min, discard the supernatant to collect the bacteria. Take 0.05g of collected bacteria, 0.0017g of β-alanine, 0.0019g of magnesium chloride, and 0.0044g of ATP and dissolve them in 2mL of pH 7 sodium phosphate buffer solution, and react in a constant temperature oscillator at 25°C, and the oscillation speed is 600~ 800r / min, add 0.0044g of histamine after the system reacts for 30min, then react for 48h.
[0084] 2. Take the reaction sample from step 1, centrifuge at 12,000 rpm for 2 minutes, take 300 μL of the supernatant, add 40 μL of NaOH (0.1M) and 600 μL of DNS (dansyl chloride, 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com