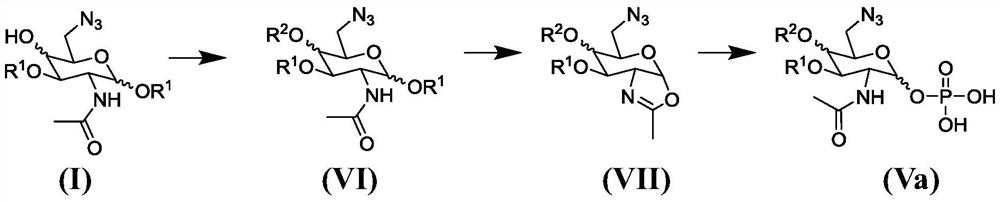
Synthesis of 6-azido-6-deoxy-2-N-acetyl-hexosamine-nucleoside diphosphate
A nucleoside diphosphate and azido-based technology, applied in the direction of sugar derivatives, sugar derivatives, esterified saccharides, etc., can solve the problems of no high yield, scalability, and incompatibility, and achieve easy access and high efficiency And yield, the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0357] Example 1-1: Synthesis of 4,6-O-benzylidene-N-acetyl-D-galactosamine
[0358]
[0359] To a suspension of N-acetyl-D-galactosamine (1299 g, 5.9 mol) in MeCN (13 L) was added DL-camphorsulfonic acid (81 g, 352 mmol) and benzaldehyde dimethyl acetal (2190 g, 2160 mL ,14.4mol). The reaction was stirred at room temperature and filtered through a Buchner funnel. The white filter cake was rinsed with MeCN (10 x 1 L) and dried on the filter overnight. Drying in a circulating oven at 35°C for four days gave the product (1938 g, 107%). By heating from EtOH / H at 5-8°C 2 Crystallization in O (13:1-9:1) for 16-24 hours afforded the pure product as a white crystalline solid. 1 H NMR (400MHz, DMSO) δ (ppm) 7.49-7.46 (m, 2H), 7.38-7.34 (m, 3H), 5.56 (s, 1H), 5.04-5.03 (m, 1H), 4.14-4.13 (m ,1H), 4.08-3.94(m,3H), 3.85-3.78(m,2H), 1.82(s,3H).
Embodiment 1-2
[0360] Example 1-2: Synthesis of 1,3-di-O-acetyl-4,6-O-benzylidene-N-acetyl-D-galactosamine
[0361]
[0362] The starting material 4,6-O-benzylidene-N-acetyl-D-galactosamine (1938 g, 6.3 mmol) was dissolved in pyridine (7750 mL) and acetic anhydride (1919 g, 1.765 L ,18.8mol). The reaction mixture was stirred overnight at room temperature, then ice water (19.5 L) was added. After stirring for 15 minutes, another scoop of ice was added, and after a further 15 minutes, the mixture was filtered. The filter cake was washed with ice water (3 x 6 L) and dried on the filter overnight, then in a circulating oven at 45°C overnight. The crude product was dissolved in methanol (13.7 L) and the mixture was heated to reflux to obtain a clear solution. The solution was cooled to room temperature overnight, then cooled to 0 °C in an ice bath for 4 hours, then filtered. The filter cake was washed 3 times with the filtrate and dried on the filter for 1.5 hours. Drying in a circulating o...
Embodiment 1-3
[0363] Example 1-3: Synthesis of 1,3-di-O-acetyl-N-acetyl-D-galactosamine (2)
[0364]
[0365] The starting material 1,3-di-O-acetyl-4,6-O-benzylidene-N-acetyl-D-galactosamine (230 g, 585 mmol) was dissolved in MeOH / dioxane (1:1, 3 L), placed in a Parr vessel and added AcOH (1.9 g, 1.77 mL, 31 mmol). Subsequently, Pd-C (37.3 g, 175 mmol) was added and the vessel was mounted on a Parr apparatus. reactant in H 2 Stir overnight under atmosphere (5 bar). Then, the mixture was filtered through a pad of celite and washed with 1,4-dioxane (1200 mL). Acetic acid (12 mL) was added to the filtrate, then concentrated under reduced pressure to give product 3 (224 g, 126%). 1 H NMR (400MHz, DMSO) δ (ppm) 7.91 (d, J = 8.4Hz, 1H), 5.96 (d, J = 3.6Hz, 1H), 5.20 (d, J = 5.2Hz, 1H), 4.84 (dd ,J=8.8,2.8Hz,1H),4.49-4.43(m,1H),3.79(t,J=6.4Hz,1H),3.55-3.50(m,1H),3.44-3.40(m,1H), 2.11(s,3H), 2.01(s,3H), 1.78(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com