Abacavir, lamivudine and efavirenz compound tablet and preparation method thereof
A technology of efavirenz and lamivudine, applied to non-active ingredient medical preparations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve poor patient compliance, poor tablet uniformity, etc. problems, to achieve the effect of good uniformity of tablets, less amount of excipients, and excellent uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
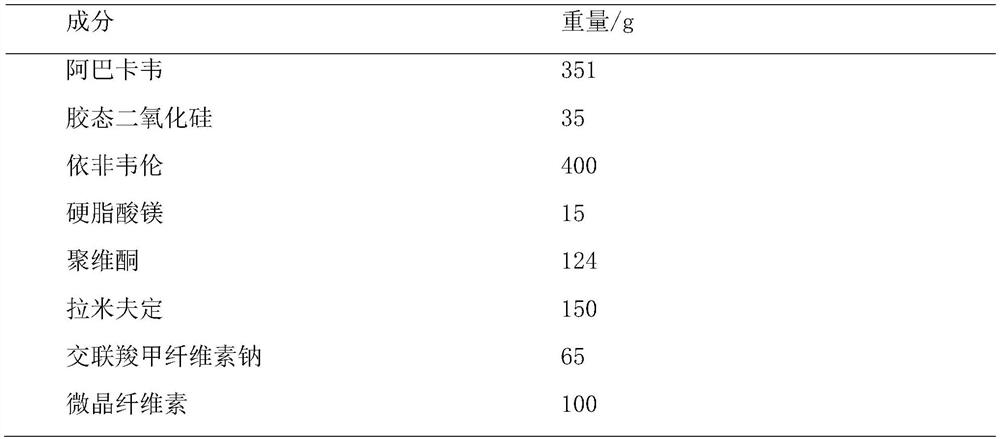
[0029] A compound tablet of abacavir, lamivudine and efavirenz, which comprises the following components:
[0030]
[0031] Preparation process: S1, preparation of the first particle
[0032] 1.1. Dissolve 60g of povidone with 30% ethanol at a ratio of 1:15 (W / V), add 200g of efavirenz and 15g of magnesium stearate to a constant temperature bath, control the temperature at 50°C, and stir at 120rpm for 10min for bonding agent, spare;
[0033] 1.2. Feed 351g of abacavir and 35g of colloidal silicon dioxide with D90≤25μm into the granulation chamber, pass in dry hot air at 62°C, and adjust the frequency of the air conditioner to 40HZ to keep the boiling height at about 77cm;
[0034] 1.3. Adjust the pressure of the compressed air entering the spray gun to 0.37MPa and the temperature to 57°C; turn on the liquid spray, transport the binder in step S1.1 to the granulation chamber for boiling granulation, and control the flow rate of the binder to 4.5L / h;
[0035] 1.4. After l...
Embodiment 2
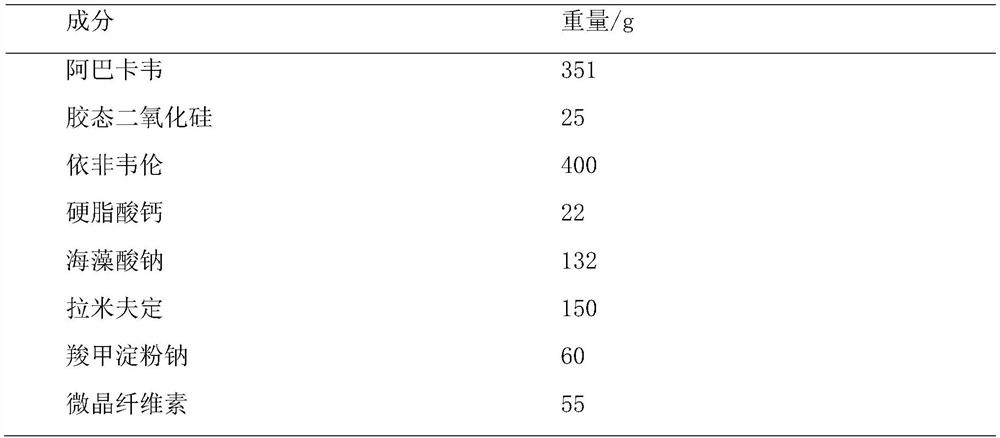
[0041] A compound tablet of abacavir, lamivudine and efavirenz, which comprises the following components:
[0042]
[0043] Preparation Process:
[0044] S1. Preparation of the first particles
[0045] 1.1. Dissolve 72g of sodium alginate with 15% ethanol at a ratio of 1:10 (W / V), add 240g of efavirenz and 22g of calcium stearate to a constant temperature tank, control the temperature at 55°C, and stir at 100rpm for 15min as a bonding agent agent, spare;
[0046] 1.2. Add 351g of abacavir into 1500ml of 55% ethanol solution (V / V) and stir vigorously to dissolve it, add 25g of colloidal silicon dioxide with D90≤25μm, stir at 30rpm for 20min, dry under reduced pressure, pulverize, pass through 80 Prepare solid dispersion after mesh sieve, standby;
[0047] 1.3. Put the above solid dispersion into the granulation chamber evenly, pass in dry hot air with a temperature of 65°C, adjust the frequency of the fan to 38HZ to maintain a boiling height of 75cm; adjust the pressure o...
Embodiment 3
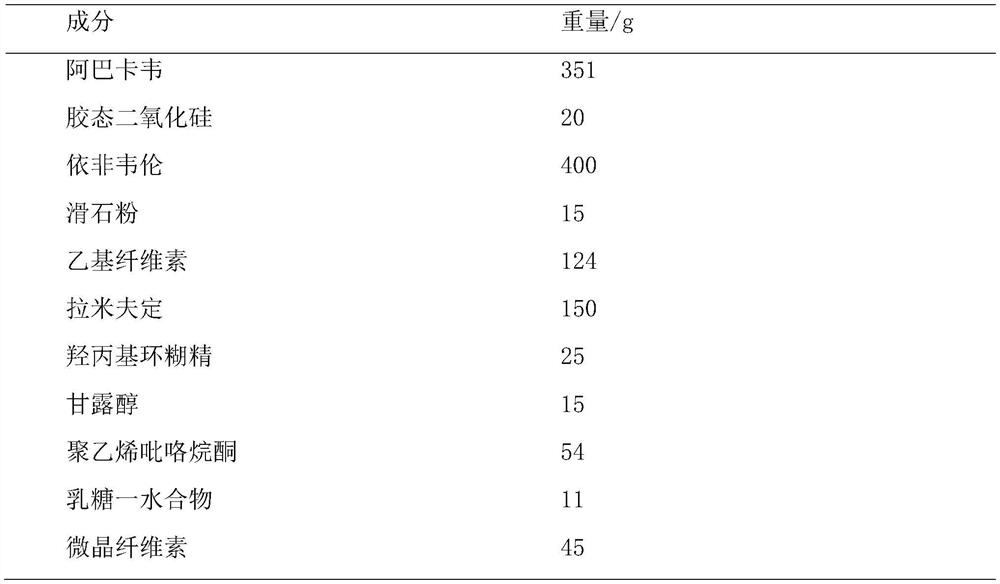
[0054] A compound tablet of abacavir, lamivudine and efavirenz, which comprises the following components:
[0055]
[0056] Prepared by the following process:
[0057] S1. Preparation of the first particles
[0058] 1.1. Dissolve 60g of ethyl cellulose with 18% ethanol at a ratio of 1:13 (W / V), add 210g of efavirenz and 15g of talcum powder and transport it to a constant temperature tank, control the temperature at 58°C, and stir at 110rpm for 12min as a binder ,spare;
[0059] 1.2. Add 351g of abacavir into 1250ml of 60% ethanol solution (V / V) and stir vigorously to dissolve it, add 20g of colloidal silicon dioxide with D90≤25μm, stir at 40rpm for 15min, dry under reduced pressure and pulverize for 80 Prepare solid dispersion after mesh sieve, standby;
[0060] 1.3. Put the above solid dispersion into the granulation chamber evenly, pass in dry hot air with a temperature of 64°C, adjust the frequency of the fan to 37HZ to maintain a boiling height of 73cm; adjust the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com