L-pantoic acid lactone dehydrogenase, mutant and application of L-pantoic acid lactone dehydrogenase
A technology of pantolactone and ester dehydrogenase, which is applied in the biocatalytic preparation of D-pantothenic acid precursor intermediate ketopantolactone, L-pantolactone dehydrogenase TpLPLDH, mutation In the field of body and its coding gene, it can solve the problems of low enzyme activity, serious inclusion body, incomplete transformation of L-pantoate lactone, etc., and achieve the effect of good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Construction of L-pantolactone dehydrogenase engineering bacteria
[0031] The amino acid sequence of L-pantolactone dehydrogenase is shown as SEQ ID NO.1, and the codon-optimized gene SEQ ID NO.3 is constructed on the expression vector pET 28a, and a 6*his tag is added to the C-terminal for subsequent protein expression. The build process is as follows:
[0032] 1. Use F-1 and R-1 as primers (Table 1) to Tp LPLDH is a template to expand gene fragment 1;
[0033] 2. Use F-2 and R-2 as primers (Table 1), and use pET 28a as a template to expand vector fragment 2;
[0034] 3. Purify Fragment 1 and Fragment 2, and use NcoI and XhoI to digest the purified Fragment 1 and Fragment 2;
[0035] 4. Ligate the purified fragment 1 and fragment 2 with T4 ligase ligase, and transform the enzyme-linked product into E. coli BL21 competent cells;
[0036] 5. Pick a single colony and culture it overnight in a liquid medium containing 50 μg / μl kanamycin at 37 °C in a s...
Embodiment 2
[0039] Example 2: Construction of Mutant Library and Selection of Mutants
[0040] For the expression plasmid pET 28a- Tp LPLDH was used as a template, and the alanine at position 29 was saturatingly mutated. The build process is as follows:
[0041] 1. Use 25-29-F and 25-29-R in the following table 2 as primers, use pET 28a- Tp LPLDH is used as a template to expand the entire plasmid containing the mutated gene;
[0042] 2. Reaction system: PCR reaction system (25 µL): 1 µL forward primer (100 µM), 1 µL reverse primer (100 µM), 12.5 µL 2×Phanta buffer, 0.5 µL dNTP mixture (10 mM each) , 1 µL plasmid template, 0.5 µL DNA polymerase Phanta (Novazyme, China) and 8.5 µL ultrapure water;
[0043] 3. Reaction program: Pre-denaturation at 95°C for 5 min, followed by 30 cycles (denaturation at 95°C for 15 s, annealing at 55°C for 15 s, extension at 72°C for 7 s), final extension at 72°C for 10 min, and incubation at 16°C;
[0044] 4. Add 1 µL DpnI (NEB, USA) and 2.5 µL Buffer ...
Embodiment 3
[0055] Embodiment 3: Mutant substrate catalytic ability test
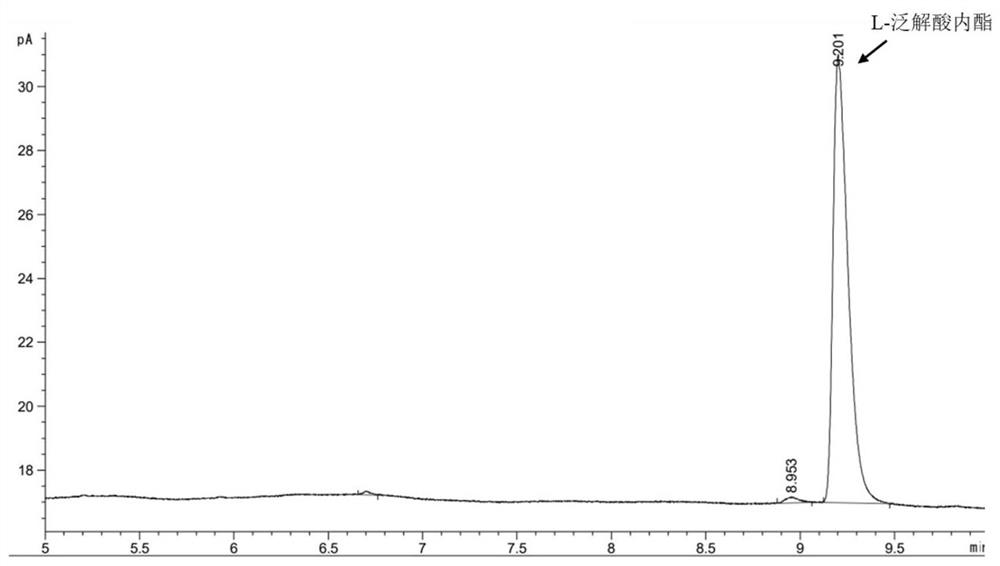
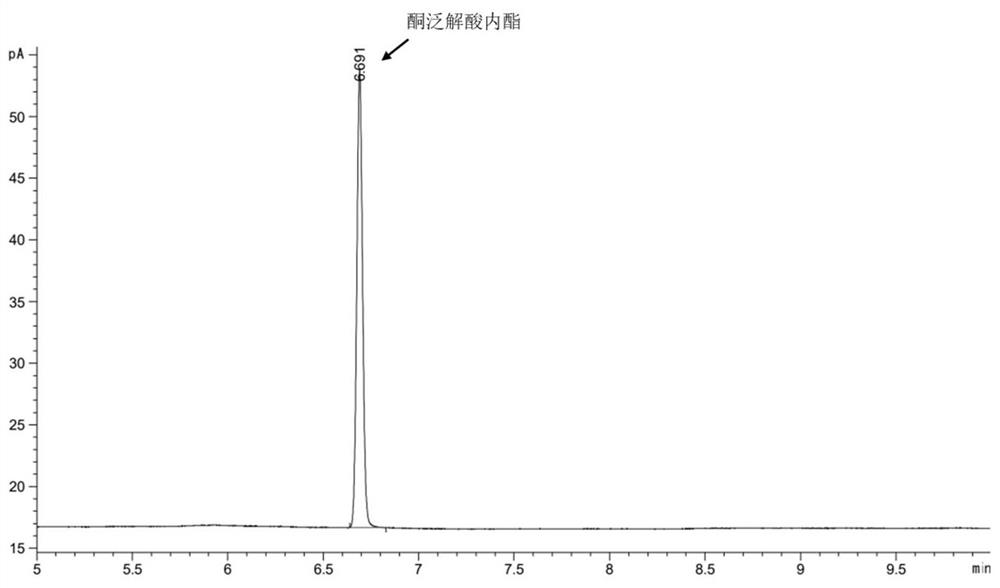
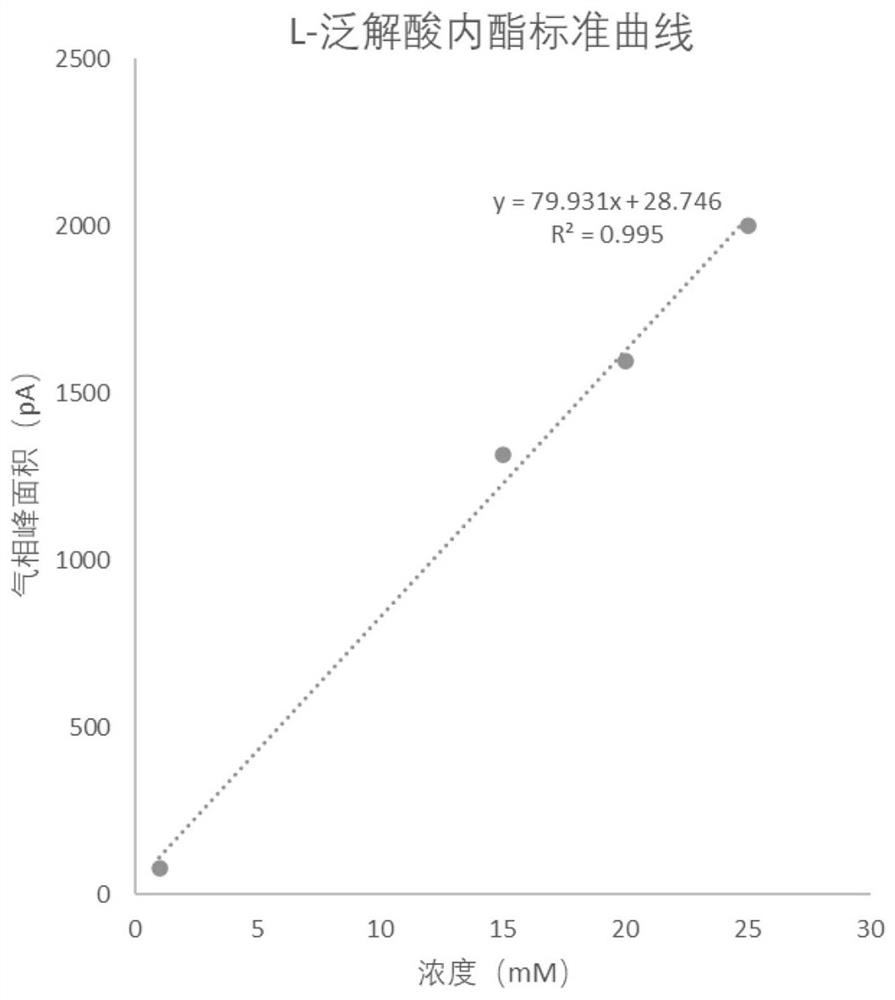
[0056] Pick the engineering strain of embodiment 1 E. coli BL21(DE3) / pET-28b- Tp LPLDH and the mutant strain constructed in Example 1 E. coli BL21(DE3) / pET-28b- Tp LPLDH A29S A single colony was cultured in LB medium containing 50 μg / μL kanamycin at 37 °C in a shaker at 180 rpm overnight. Transfer the seed solution to 100 mL LB liquid medium containing 50 μg / μL kanamycin at 10% inoculum size, and culture it in a shaker at 37 °C and 180 rpm for 2-2.5 h to make the OD of the strain 600 Reach between 0.6-0.8. Add a final concentration of 0.1 mM isopropylthiogalactopyranoside (Isopropyl β -D-thiogalactoside, IPTG) at 28°C, 180 rpm for 12 h. then at 4 o C. Centrifuge at 12000rpm for 10 min to obtain protein containing L-pantolactone dehydrogenase activity Tp LPLDH and Tp LPLDH A29S wet bacteria;
[0057] Each strain was treated with pH 7.0, 50 mM phosphate buffer (0.2 M Na 2 HPO 4 , 0.2 M NaH 2 PO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com