Bipyridine ligand containing AIE and ACQ groups, amphiphilic rhombic supramolecular metal ring and application of amphiphilic rhombic supramolecular metal ring
A bipyridine and amphiphilic technology, applied in the field of pharmaceutical preparations, can solve the problems of inapplicability for biological imaging, increase water solubility and biocompatibility, etc., and achieve good coordinated fluorescence emission, convenient cell imaging, and clear cell imaging effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1. Preparation of 120° Bipyridine Ligands Containing Aggregation-Induced Emission (AIE) and Aggregation Fluorescence Quenching (ACQ) Groups
Embodiment 1-1
[0117] Example 1-1: Bispyridine ligand 1a
[0118]
[0119] Step (1), the preparation of compound 6:
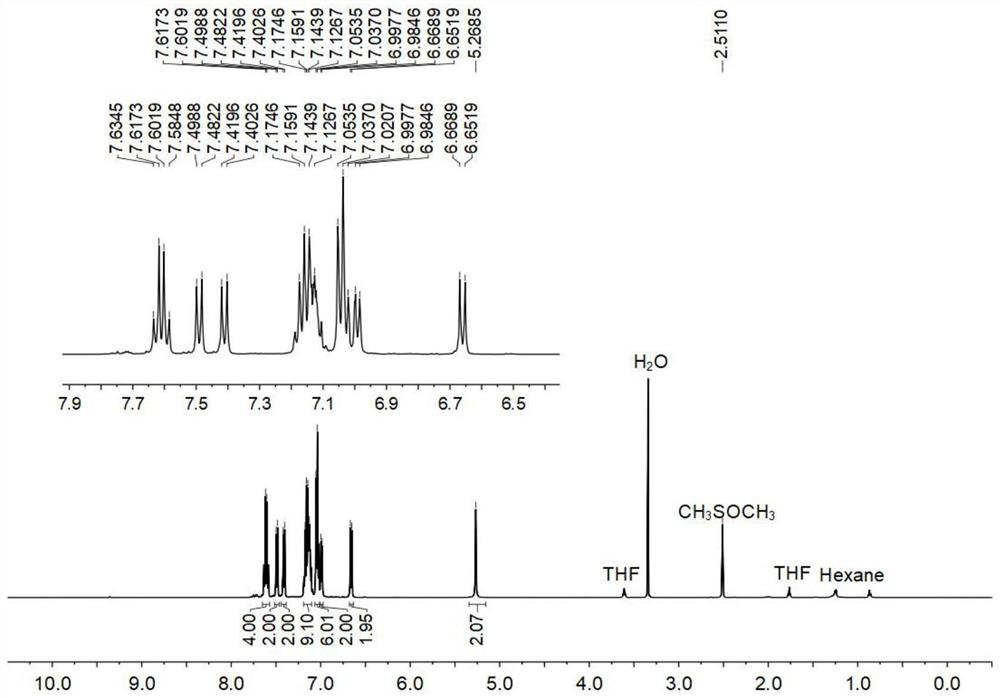
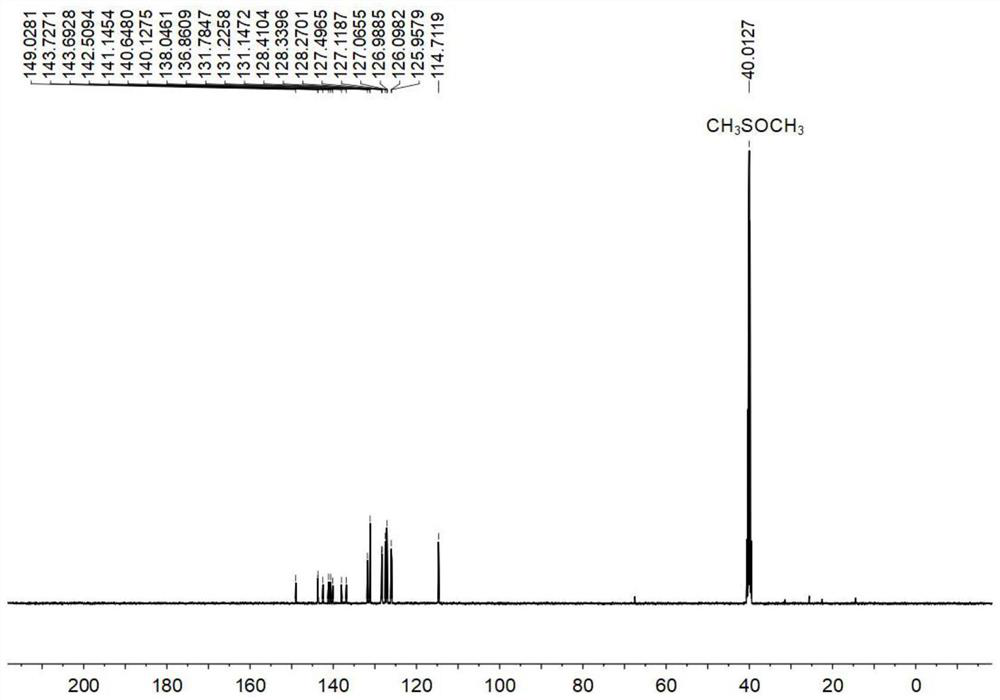
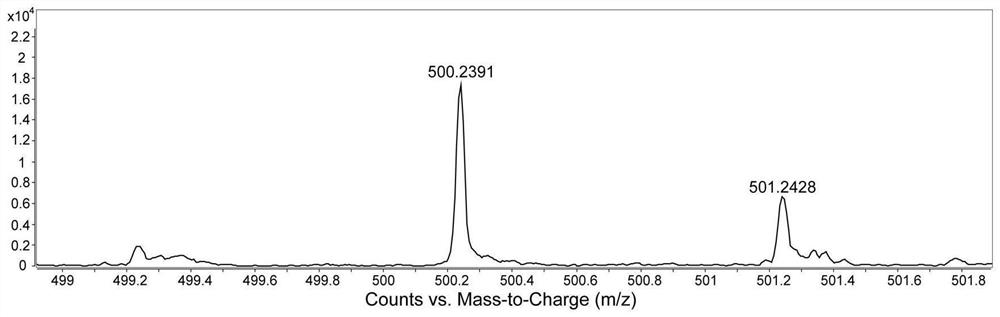
[0120] Compound 4 (1000.0mg, 5.61mmol), compound 5 (1139.8mg, 3.03mmol), potassium carbonate (1396.0mg, 10.10mmol), tetrakis (triphenylphosphine) palladium (26.4mg, 0.02mmol) was added to a 250mL Add a mixed solvent (1,4-dioxane / water, 4 / 1, v / v) to the Schlenk bottle to dissolve, then use liquid nitrogen and nitrogen gas to deoxygenate three times, and store at 80°C under the protection of argon The reaction was heated for 48 hours. After the reaction was finished, cool to room temperature, concentrate under reduced pressure with a rotary evaporator, and extract three times with dichloromethane, collect the organic phase, dry and filter to obtain the crude product, which was separated and purified by silica gel chromatography (petroleum ether / ethyl acetate, 6 / 1, v / v), finally obtained a yellow solid (705.0mg, 43.4%), melting point: 247.7-250.1°C.
[0121] Figure 1-3 S...
Embodiment 1-2
[0128] Example 1-2: Bispyridine ligand 1b
[0129]
[0130] Step (1), the preparation of compound 6:
[0131] Compound 4 (1000.0mg, 5.61mmol), compound 5 (1139.8mg, 3.03mmol), potassium carbonate (1396.0mg, 10.10mmol), tetrakis (triphenylphosphine) palladium (26.4mg, 0.02mmol) was added to a 250mL Add a mixed solvent (1,4-dioxane / water, 4 / 1, v / v) to the Schlenk bottle to dissolve, then use liquid nitrogen and nitrogen gas to deoxygenate three times, and store at 80°C under the protection of argon The reaction was heated for 48 hours. After the reaction was finished, cool to room temperature, concentrate under reduced pressure with a rotary evaporator, and extract three times with dichloromethane, collect the organic phase, dry and filter to obtain the crude product, which was separated and purified by silica gel chromatography (petroleum ether / ethyl acetate, 6 / 1, v / v), finally obtained a yellow solid (705.0mg, 43.4%), melting point: 247.7-250.1°C.
[0132] Figure 1-3 S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com