Method for recovering fluoride salt from complex aluminum electrolyte
An aluminum electrolyte and fluoride salt technology, applied in chemical instruments and methods, aluminum fluoride, aluminum compounds, etc., can solve the problems of high electrolyte recovery cost, environmental pollution, poor operation stability of aluminum electrolytic cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
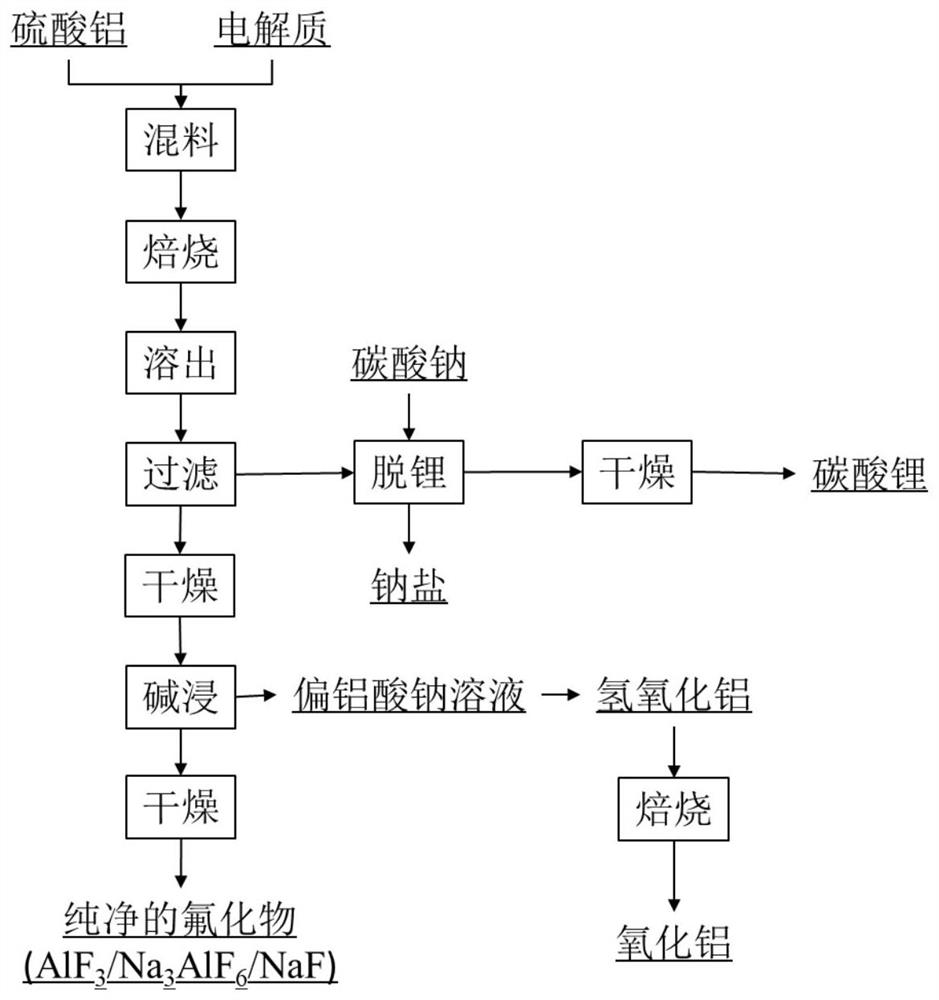
[0027] S1: Mix the crushed complex aluminum electrolyte and aluminum sulfate evenly, and perform a roasting reaction to obtain roasted material A. The mass ratio of the complex aluminum electrolyte to aluminum sulfate is 1:0.5, the calcination temperature is 400°C, and the calcination time is 60min.
[0028] S2: pickling and washing the calcined material A obtained in step S1, the acid used for pickling is dilute sulfuric acid with a mass concentration of 5%; the washing process is carried out until the pH of the washing solution is 7. Then filter to obtain filtrate A and filter residue A.
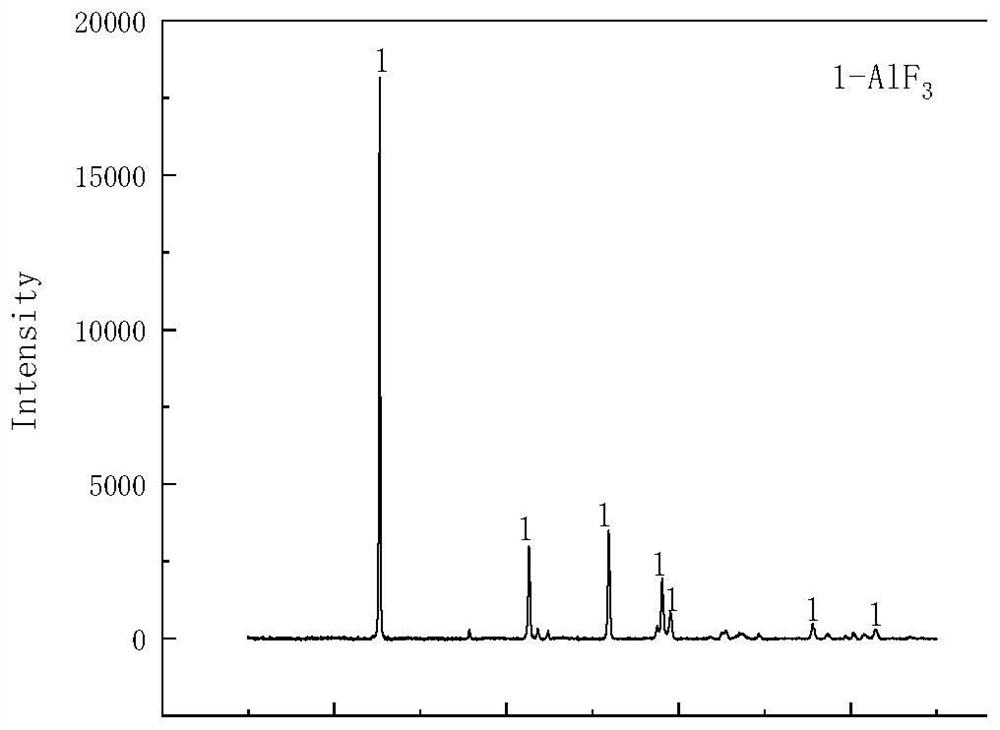
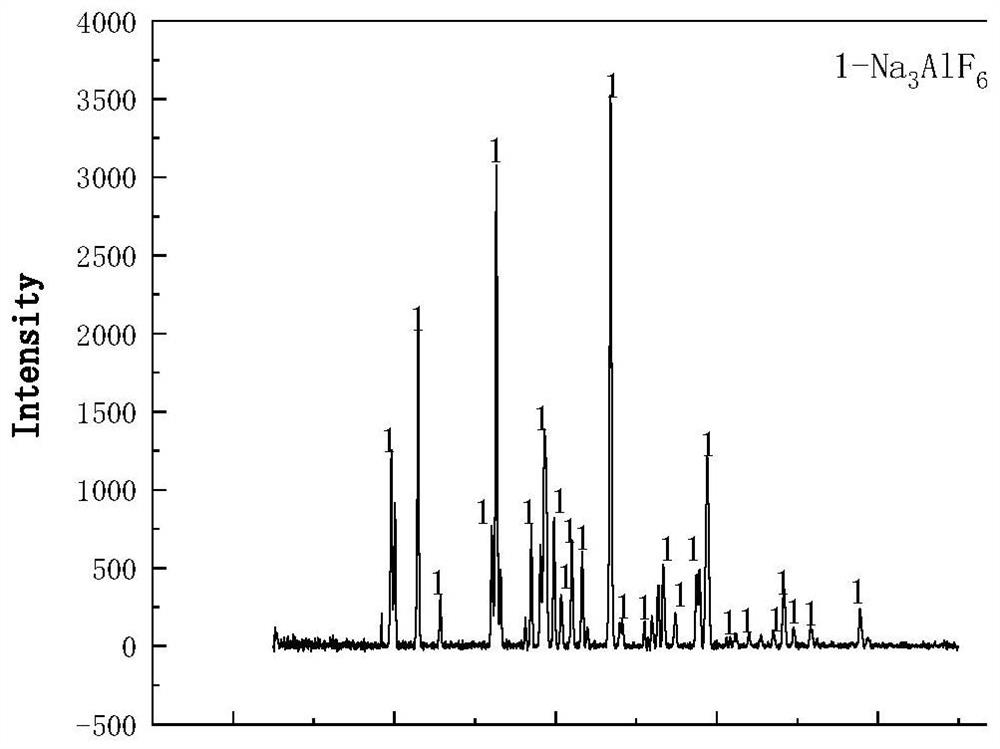
[0029] S3: After the filter residue A is dried at 60°C, it is added to the alkali solution (sodium hydroxide solution with a mass percentage of 20%), the mass ratio of the dried filter residue to the alkali solution is 1:1.5, and it is dried in an oven at 40°C Alkali leaching in the medium, the alkali leaching time is 40min. Afterwards, filtrate B and filter residue B were obtained by fi...
Embodiment 2
[0032] Basically the same as Example 1, the difference is that the filtrate B produced in step S3 is also processed to prepare alumina:
[0033] S4: React the filtrate B in step S3 with an acid to obtain aluminum hydroxide, wherein the acid reacted with the filter residue B is a dilute sulfuric acid solution with pH=5, and the temperature for the reaction between the filtrate B and the acid is 40°C.
[0034] The obtained aluminum hydroxide is roasted at a temperature of 800° C. to further obtain alumina products.
Embodiment 3
[0036] Substantially the same as Example 1, the difference is that the filtrate A in S2 is also included in the recovery of Li:
[0037] S5: Add enough sodium carbonate to the filtrate A obtained in step S2 to heat and stir, and the heating temperature is 50°C. Filtrate C and filter residue C are obtained by filtration, and the main component of filter residue C is lithium carbonate, which can be used as a raw material for the production of high-purity lithium carbonate.
[0038] The filtrate C was evaporated and crystallized at 60°C to obtain sodium sulfate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com