Preparation method of ferronickel catalyst for hydrogen production through water electrolysis
A technology for catalyst and water electrolysis, applied in the electrolysis process, electrolysis components, electrodes, etc., can solve the problems of unfavorable large-scale production, easy detachment of catalyst, long reaction time, etc., and achieve good catalytic performance and stability, uniform morphology and size , the effect of shortened response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
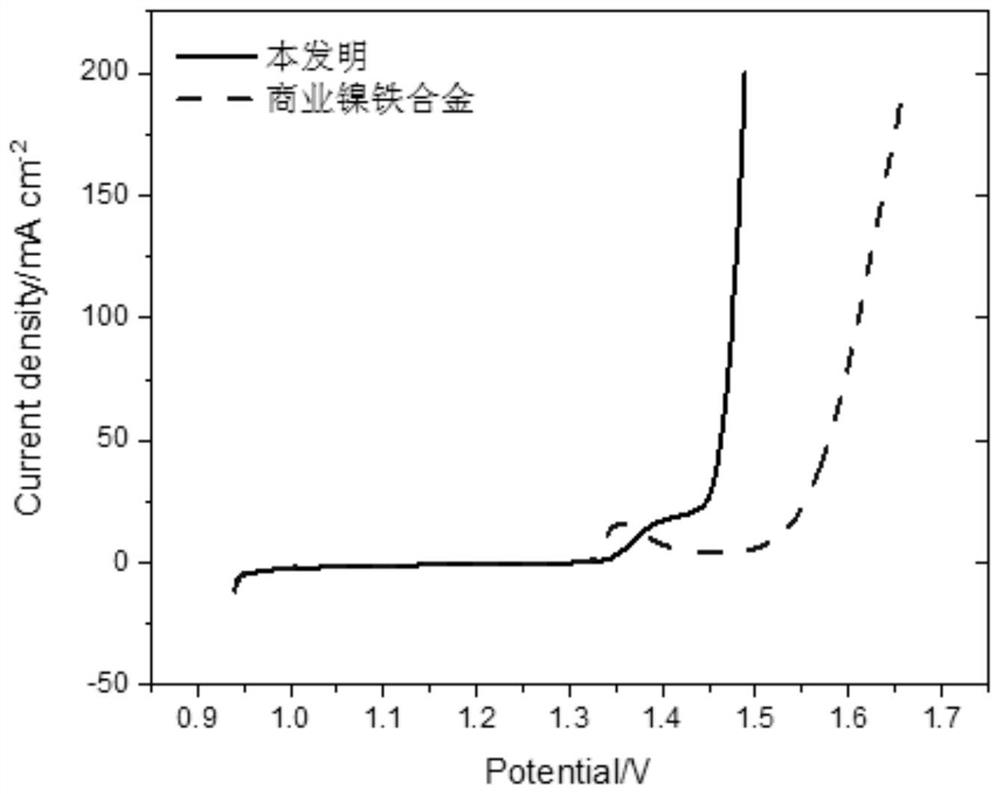
Image
Examples
Embodiment 1
[0033] First, pre-treat the nickel mesh, cut the nickel mesh into nickel sheets with a size of 5cm×5cm, wash and degrease with 1M sulfuric acid, and sonicate for 15 minutes; then ultrasonicate the acid-treated nickel mesh with ethanol and ultrapure water for 15 minutes, and finally Vacuum dry at 70°C for 25min. Secondly, add nickel sulfate hexahydrate and ferrous sulfate heptahydrate into water and dissolve to obtain a solution, the concentration of nickel sulfate in the solution is 0.75 moles per liter, the concentration of ferrous sulfate is 0.15 moles per liter, and the total volume of the solution is 200 mL . Add 4g of sodium chloride, 9g of boric acid, 6g of sodium citrate, 0.08g of benzene sulfinic acid agent, 0.06g of sodium lauryl sulfate, and 1g of saccharin into the above-mentioned dissolving solution in turn, and make it dissolve, and mix well to obtain Dark green transparent mixed solution. Then, use a pH meter (with stirring) to adjust the pH of the above soluti...
Embodiment 2
[0035] First, pre-treat the nickel mesh, cut the nickel mesh into nickel sheets with a size of 5cm×5cm, wash and degrease with 1M sulfuric acid, and sonicate for 15 minutes; then ultrasonicate the acid-treated nickel mesh with ethanol and ultrapure water for 15 minutes, and finally Vacuum dry at 70°C for 25min. Next, add nickel sulfate hexahydrate and ferrous sulfate heptahydrate into water for dissolution to obtain a solution, the concentration of nickel sulfate in the solution is 0.8 moles per liter, the concentration of ferrous sulfate is 0.15 moles per liter, and the total volume of the solution is 200 mL . Add 4g of sodium chloride, 9g of boric acid, 6g of sodium citrate, 0.08g of benzenesulfinic acid agent, 0.06g of sodium lauryl sulfate, and 1g of saccharin into the above-mentioned dissolving solution, dissolve them, and mix them uniformly to obtain ink. Green transparent solution. Then, use a pH meter (with stirring) to adjust the pH of the above solution to 3, and t...
Embodiment 3
[0037]First, pre-treat the nickel mesh, cut the nickel mesh into nickel sheets with a size of 5cm×5cm, wash and degrease with 1M sulfuric acid, and sonicate for 15 minutes; then ultrasonicate the acid-treated nickel mesh with ethanol and ultrapure water for 15 minutes, and finally Vacuum dry at 70°C for 25min. Secondly, add nickel sulfate hexahydrate and ferrous sulfate heptahydrate into water and dissolve to obtain a solution, the concentration of nickel sulfate in the solution is 0.75 moles per liter, the concentration of ferrous sulfate is 0.1 moles per liter, and the total volume of the solution is 200 mL . Then add 3g of sodium chloride, 10g of boric acid, 5g of sodium citrate, 0.06g of benzene sulfinic acid agent, 0.08g of sodium lauryl sulfate, and 1g of saccharin into the above-mentioned dissolving solution, and make it dissolve, and mix well to obtain Dark green transparent mixed solution. Then, use a pH meter (with stirring) to adjust the pH of the above solution t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com