A fusion protease and its preparation method, its application in extracting type I collagen and the use of the type I collagen
A collagen and protease technology, applied in the field of biogenetic engineering, can solve the problem of destroying the spatial structure of type I collagen, and achieve the effects of promoting epithelial cell proliferation, promoting collagenase production, excellent biocompatibility and biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 protease
[0052] 1. Optimizing the catalytic domains of protease 7 and protease 9
[0053]On the REACTOME website, find out the protease 7 and protease 9 in the rat tail tendon of the mouse, and perform structural analysis on the protease 7 and protease 9 in the BRENDA, MEROPS, and UNIPROT databases. Find the sequence of the catalytic domain in the UNIPROT database, and optimize the sequence of the catalytic domain according to the preference of E. coli for amino acids. The sequence of the optimized protease 7 (mmp7c) is shown in the sequence table SEQ ID NO.4. The sequence of protease 9 (mmp9c) is shown in SEQ ID NO.5 of the sequence listing.
[0054] 2. Construction of recombinant plasmids and recombinant strains
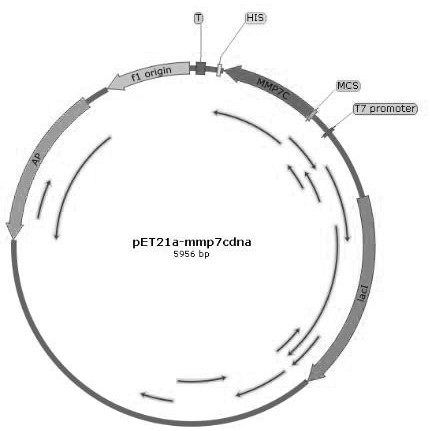
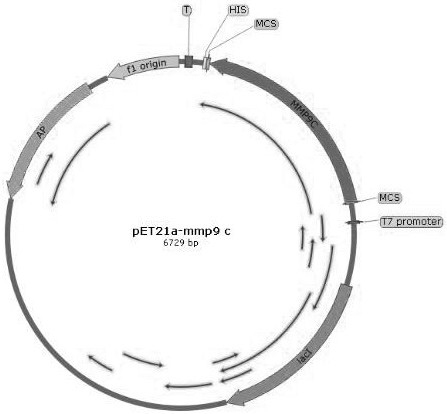
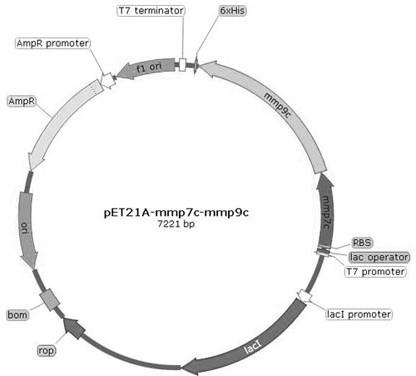
[0055] The synthesized sequence and the plasmid pET21a were digested, and the sequences of mmp7c, mmp9c, mmp7C-linker-mmp9C were respectively constructed on the plasmid pET21a to obtain pET21a-mmp7c, pET21a-mmp9c and pET21...
Embodiment 2
[0106] Example 2 Detection and Analysis of Protease on Rat Tail Tendon Degradation
[0107] Divide the experiment into 10 groups, respectively numbered A1, A2, B1, B2, C1, C2, D1, D2, E1, E2, take 1g of fresh rat tail tendon, soak in 1:1000 bromogeramine solution for 10-15min After taking it out, rinse it with normal saline for more than 5 times, pour off the normal saline, and trim it to 1mm with scissors 3 The left and right tissue pieces were put into the Erlenmeyer flask, and 100ml of 0.05M Tris-Hcl (pH7.5) solution containing 1M Nacl was added, and left for 1 day. After 1 day, discard the supernatant. Add an appropriate amount of normal saline to A1 and A2, add an equal amount of acetic acid solution and trypsin solution to B1 and B2, add an equal amount of protease 7 solution to C1 and C2, and add an equal amount of protease to D1 and D2 9 solution, add an equal amount of fusion protease to E1 and E2, extract at 37°C for 8 hours, shake and observe regularly. Then cent...
Embodiment 3
[0113] Example 3 Characteristic Detection of Extracting Type I Collagen from Rat Tail Tendon Using Fusion Protease
[0114] 1. Moisture retention test: After the samples were freeze-dried, their initial moisture mass fractions were determined by the oven method. Using glycerol as a reference, freeze-dried samples were placed in a desiccator with a relative humidity of RH=81% for about 80 hours, and the relationship between their dilution rate and time was measured. Dilution rate = [(W 1 -W 0 ) / W 0 ]*100% W 0 , W 1 are the mass of the sample before placing and after placing for a certain period of time, respectively. After the moisture absorption is completed, the sample is transferred to a calcium chloride desiccator, and the relationship between the residual moisture rate of the sample and time is determined. Moisture residual rate = [(W 2 -W 0 ) / W 0 ]*100% W 0 Same as moisture absorption test; W 2 is the mass of the sample after standing for a certain period of ti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com