Freeze-drying protective agent of glycosaminoglycan synthetase and application of freeze-drying protective agent
A technology of freeze-drying protective agent and glycosaminoglycan, which is applied in the field of freeze-dried preparation of glycosaminoglycan synthetase and its preparation, can solve the problems such as no effect found, achieve simple formula, facilitate transportation and long-term storage, good The effect of resolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare the purified enzyme solution of heparin synthase I for the synthesis of heparin and heparan sulfate, the steps are as follows:
[0034] 1) Construction of engineering strains
[0035] For the specific construction process of strain BL21(DE3)-GaKfiA, please refer to the literature "The second member of the bacterial UDP-N-acetyl-D-glucosamine: heparosan alpha-1,4-N-acetyl-D-glucosaminyltransferase superfamily: GaKfiA from Gallibacterium anatis".
[0036] 2) Strain recovery and fermentation culture
[0037] Take out the cryopreservation tube of BL21(DE3)-GaKfiA strain in the -80℃ refrigerator and inoculate it into LB solid medium (containing 100 μg / mL kanamycin) for activation, pick a single colony in LB (containing 100 μg / mL kanamycin) ) seed medium (37°C, 220r / min), transfer the seed solution to 1L LB (containing 100μg / mL kanamycin) liquid medium for expansion and culture, and when the OD is about 0.6, add the final The concentration was 0.5mM IPTG, induced at ...
Embodiment 2
[0041] Prepare heparin synthase II purified enzyme solution for synthesizing heparin and heparan sulfate, the steps are as follows:
[0042] 1) Construction of engineering strains
[0043] For the construction method of strain BL21(DE3)-BtHS1, refer to patent CN113564139A.
[0044] 2) Strain recovery and fermentation culture
[0045] Take out the cryopreservation tube of BL21(DE3)-BtHS1 strain in the -80℃ refrigerator and inoculate it into LB solid medium (containing kanamycin 100 μg / mL) for activation, pick a single colony in LB (containing kanamycin 100 μg / mL) ) seed medium (37°C, 220r / min), transfer the seed solution to 1L LB liquid medium (containing 100μg / mL kanamycin) for expansion, and cultivate until the OD is about 0.6, then add the final The concentration was 0.5mM IPTG, induced at 22°C and 220r / min for 16-20h, the bacteria were collected by centrifugation, and placed in a -20°C refrigerator for later use.
[0046] 3) Purification of Heparin Synthase II
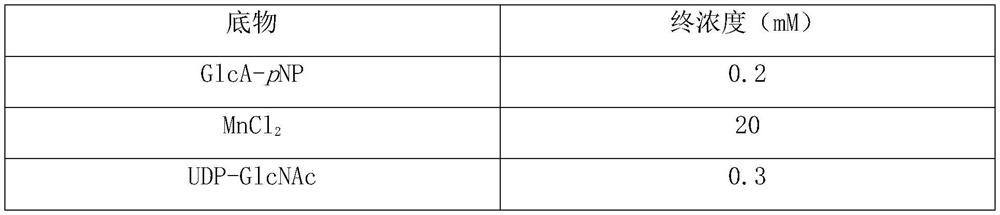
[0047] ...
Embodiment 3-9
[0049] A preparation method of a freeze-dried preparation of heparin synthase I, comprising the following steps:
[0050] 1. Dissolve ectoine, trehalose and hydroxypropyl-β-cyclodextrin with a certain amount of purified water to obtain a lyoprotectant solution, which is filtered through a sterile filter;
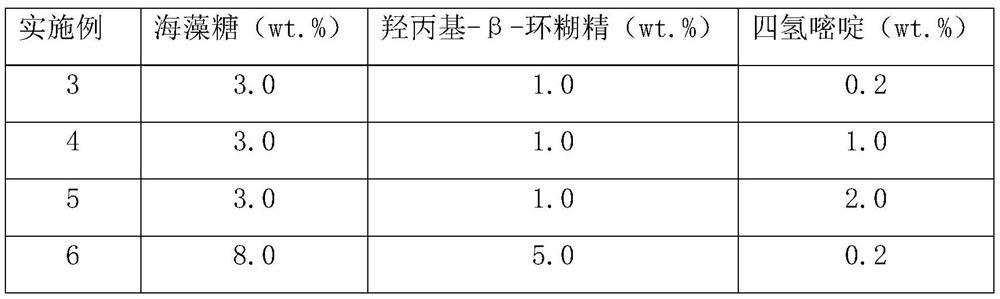
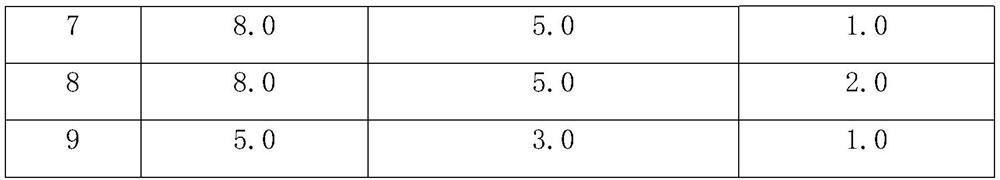
[0051] 2. Mix the lyoprotectant solution with the purified enzyme solution of heparin synthase I to obtain the total raw material solution, so that the concentration of heparin synthase I in the total raw material solution is 0.05wt.%, so that ectoine, trehalose and hydroxyl The final concentration of propyl-β-cyclodextrin in the total raw material solution meets the requirements in Table 1, and water makes up 100wt.%.
[0052] Table 1
[0053]
[0054]
[0055] Note: "wt.%" refers to the grams of ingredients contained in 100ml of liquid.
[0056] 3. Divide the total raw material solution according to the amount of 1ml / sterile vial, and then place it in a -80°C refri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com