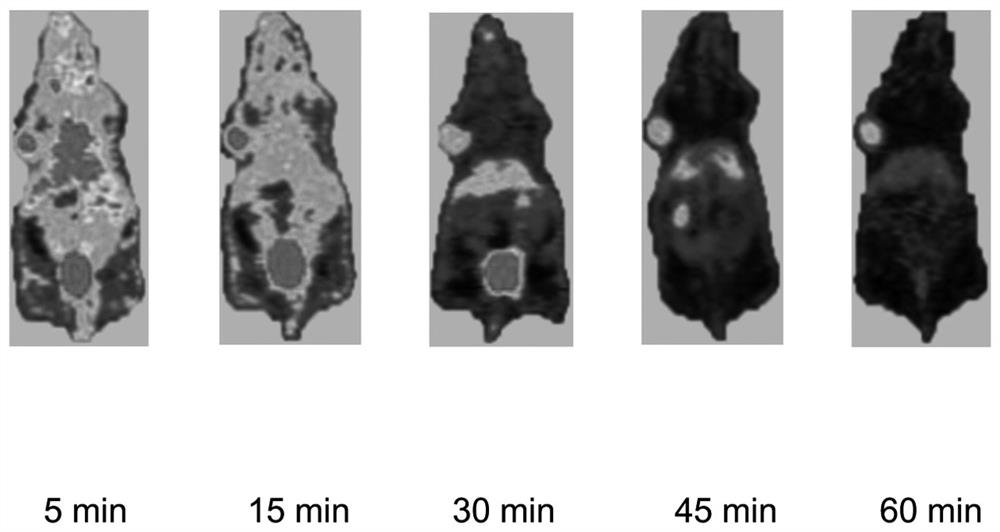
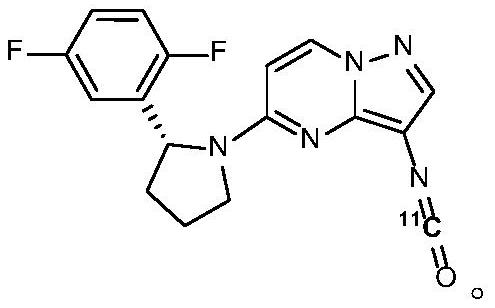
Carbon-11 labeled Larotrectinib compound and preparation method thereof
A compound and labeling technology, applied in organic chemistry, radioactive carriers, etc., can solve the problems of time-consuming and infeasible, and achieve the effects of shortening the reaction time, simple and easy operation steps, and less usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0148]
[0149] step one:[ 11 Synthesis of C]-3-(isocyanato)pyrazolo[1,5-a]pyrimidine analogue intermediate (compound 4)
[0150]
[0151] 1 milliliter of V-type reaction bottle, after nitrogen flow (10ml / min) purging 5 minutes, add 100 μ L acetonitrile, 5 μ L BEMP, 100 μ g compound 1, the [ 11 CO 2 ]CO 2 Under the purging of nitrogen flow (10mL / min), pass through the acetonitrile solution above, and pass through the small radiation detector at the proximal end, measure the radioactivity captured by the solution until it reaches the peak value. After reacting for 1 min, add 100 μl of freshly prepared POCl 3 (0.2% v / v) acetonitrile solution, reacted for 30 seconds, sampled and analyzed to obtain compound 4, which was directly used in the next reaction without purification.
[0152] Step two, [ 11 Preparation of C]-3-R-Larotrectinib Compound
[0153]
[0154] Add 100 μL of compound 2 (0.2 mg) in acetonitrile solution to the reaction mixture in step 1. After reacti...
Embodiment 6
[0160] step one:[ 11 Synthesis of C]-3-(isocyanato)pyrazolo[1,5-a]pyrimidine analogue intermediate (compound 4)
[0161] Same as Step 1 of Example 1.
[0162] Step two, [ 11 Preparation of C]-Larotrectinib Compound
[0163]
[0164] In the reaction mixture in step 1, add 100 μl of (S)-3-pyrrolidinol (0.15 mg) in acetonitrile solution, react for 1 minute, take a sample for analysis and detection, TLC traces that the labeled precursor compound 4 basically disappears, radioactive TLC traces the reaction Mixture sampling (1-2μL) was tested to have [ 11 C] - Larotrectinib labeled product. The reaction was quenched with 750 μl of ethanol / water (1 / 1, v / v). After the mixture was separated and purified by preparative HPLC (the specific process was the same as in Example 1), the eluate of the target product was collected and concentrated to dryness in vacuum at 70° C. ,get[ 11 C] - Larotrectinib compound.
Embodiment 7~12
[0166]
[0167] step one,[ 11 C]COF 2 Synthesis;
[0168]
[0169] cyclotron nuclear reaction 14 N(p,α) 11 C produces [ 11 C]CO 2 (3–4GBq), successively passed through a hot molybdenum target (875°C), a sodium hydroxide absorption bottle, and a stainless steel Erlenmeyer bottle cooled by liquid nitrogen containing 0.4g AgF 2 , the whole process takes 11-12 minutes to complete, [ 11 C]COF 2 Radiochemical yield (RCY) 71 + / - 2%. Under the condition of 5mL / min helium flow rate, 0.4g of AgF 2 can almost quantitatively [ 11 C] CO into [ 11 C]COF 2 . It was not detected in the NaOH system [ 11 C]COF 2 or[ 11 C]CO 2 , AgF 2 It can also be recycled.
[0170] References, Preparation [ 11 C] Fluorophosgene.Jimmy E.Jakobsson et al.,(2020).[11C]Carbonyldifluoride-a new and highly efficient[11C]carbonyl group transferagent.Angew.Chem.Int.Ed.,DOI: 10.1002 / anie. 201915414
[0171] Step two, [ 11 Preparation of C]-3-R-Larotrectinib Compound
[0172]
[0173] [ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com