Tri-substituted phenyl-1, 2, 4-triazole derivative as well as preparation and application thereof in treatment of neuron injury
A derivative and tri-substituted technology, applied in the field of medicinal chemistry, can solve the problems of difficult to cross the blood-brain barrier, poor clinical effect, influence effect, etc., and achieve the effects of excellent blood-brain barrier penetration, simple preparation method and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0047] Example 1 Preparation of trisubstituted phenyl-1,2,4-triazole derivatives
[0048] The structure of the trisubstituted phenyl-1,2,4-triazole derivative provided by the present invention is shown in formula I:
[0049]
[0050] In formula I: R 1 Respectively -H, -OH, -CH 2 OH,R(C 1 -C 4 ),-OR(C 1 -C 4 ),-X(F,Cl,Br),-CX 3 (F,Cl),-NO 2 ,-NH 2 ,-NR 2 (C 1 -C 4 ),-COOH,-COR(C 1 -C 4 ),-COOR(C 1 -C 4 ); 2 Respectively -H, -OH, -CH 2 OH,R(C 1 -C 4 ),-OR(C 1 -C 4 ),-X(F,Cl,Br),-CX 3 (F,Cl),-NO 2 ,-NH 2 ,-NR 2 (C 1 -C 4 ),-COOH,-COR(C 1 -C 4 ),-COOR(C 1 -C 4 ); m are 0,1,2,3 respectively; R 3 Respectively -OH, -CH 2 OH,R(C 1 -C 4 ),-OR(C 1 -C 4 ),-X(F,Cl,Br),-CX 3 (F,Cl),-NO 2 ,-NH 2 .
[0051] The present invention also includes precursors or pharmaceutically acceptable salts having the above structures. The precursors include prodrug forms such as esters and ethers, and the pharmaceutically acceptable salts include salts formed with ...
Embodiment 2
[0149] The lipophilicity calculation of embodiment 2 compound 1 to 12
[0150] SwissADME is a tool for calculating ADME parameters, pharmacokinetic properties and druggability properties of small molecules (Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of smallmolecules[J].Scientific Reports.2017,7:42717.) Import the structural formulas of compounds 1-12 on SwissADME, calculate the oil-water partition coefficient Log P of each compound, molecular polar surface area and rotatable bonds and other parameters, the results are shown in the following table 1.
[0151]Compounds with good blood-brain barrier permeability generally have the following chemical properties: molecular weight less than 450, LogP between 2 and 5; molecular polar surface area less than The number of hydrogen bond donors is less than 3, the number of rotatable bonds is less than 8, calculated by software, and the blo...
Embodiment 3
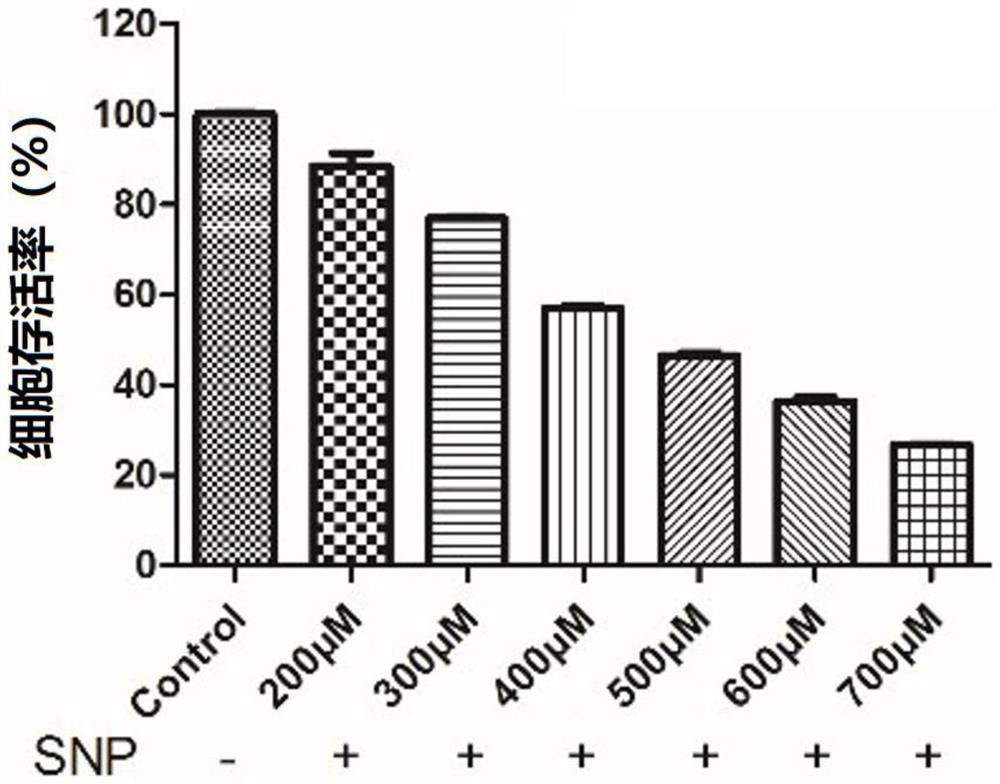
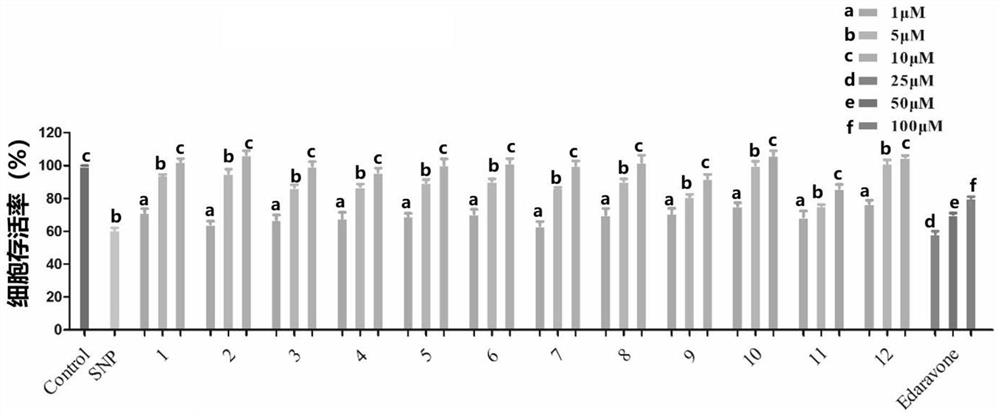
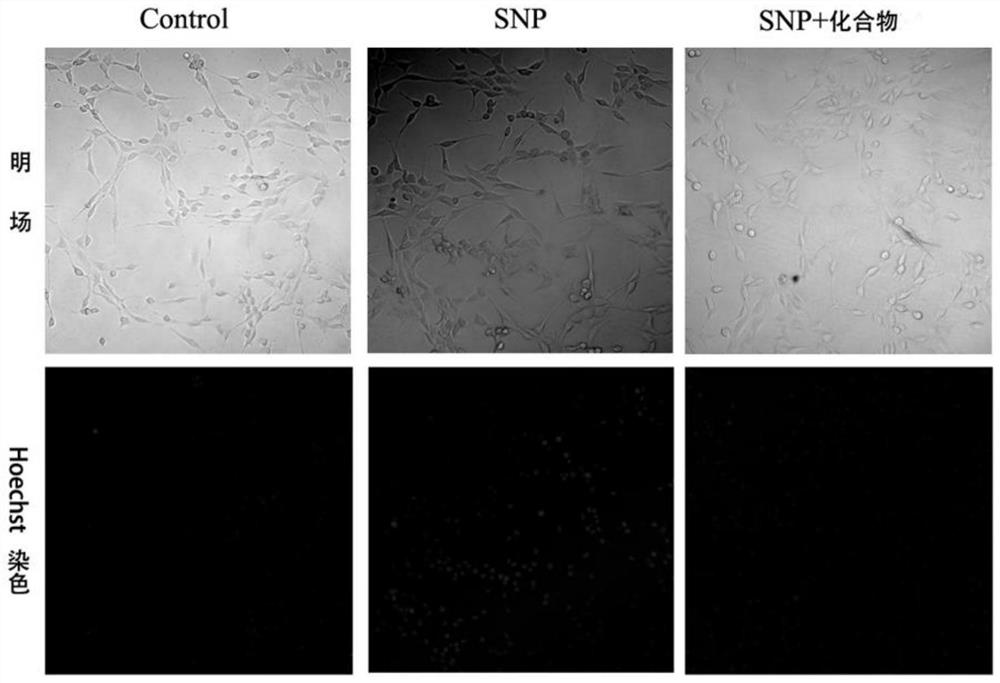
[0154] Example 3 Study on the protective effect of compound 1-12 on PC12 cell injury induced by sodium nitroprusside
[0155] (1) Establishment of injury model of PC12 cells induced by nitroprusside (SNP)
[0156] Rat adrenal pheochromocytoma cells (PC12 cells) were placed in DMEM medium containing 5% fetal bovine serum and 5% horse serum at 37°C, 5% CO 2 cultivated in an environment. After the cells adhered to the wall for a period of time, they were digested with trypsin, diluted with medium, and counted with a cell counting plate. Then, PC12 cells were inoculated in a 96-well plate at a density of 5000 cells / well, culture medium was added for 24 hours, the old medium was discarded, new medium was added to the control group to continue the culture, and the experimental group was added with different SNP concentrations ( 200, 300, 400, 500, 600 μM) culture medium. After 12h, the cell survival rate was measured by MTT method, cell survival rate=OD 各浓度 / OD control *100%, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com