Preparation method of alkyl-substituted diether type electron donor
A technology of electron donor and diether, which is applied in the field of preparation of diether electron donor, which can solve the problems of high synthesis cost and long reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
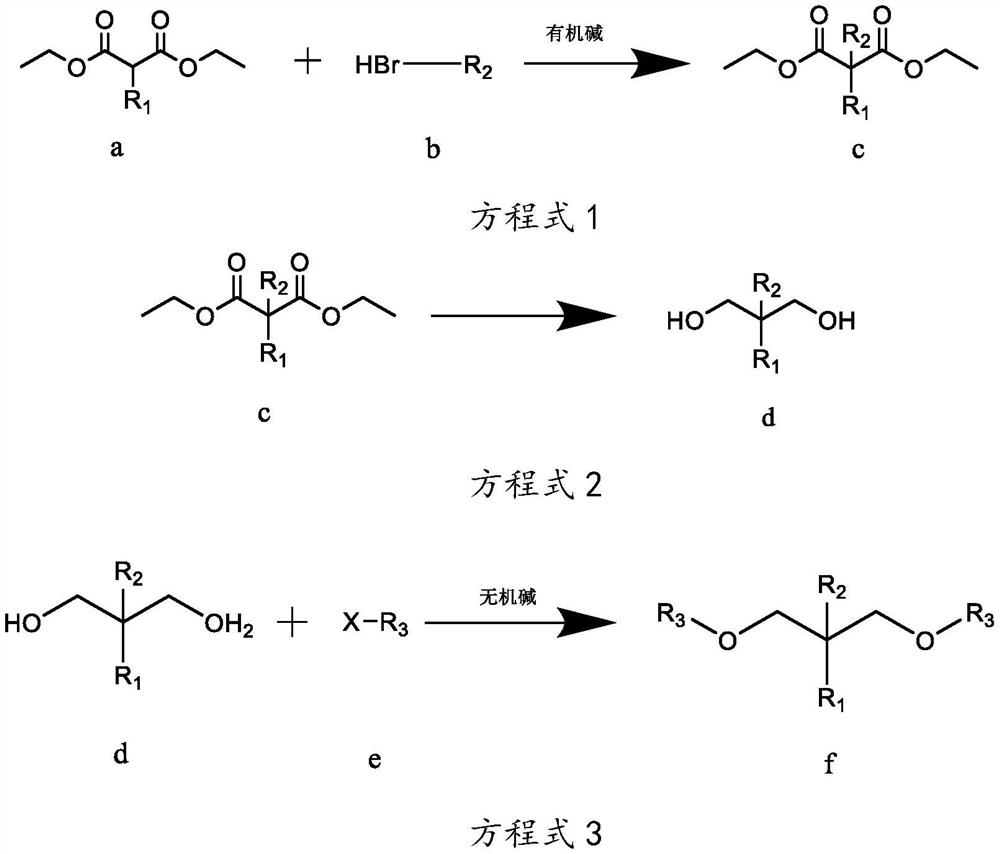
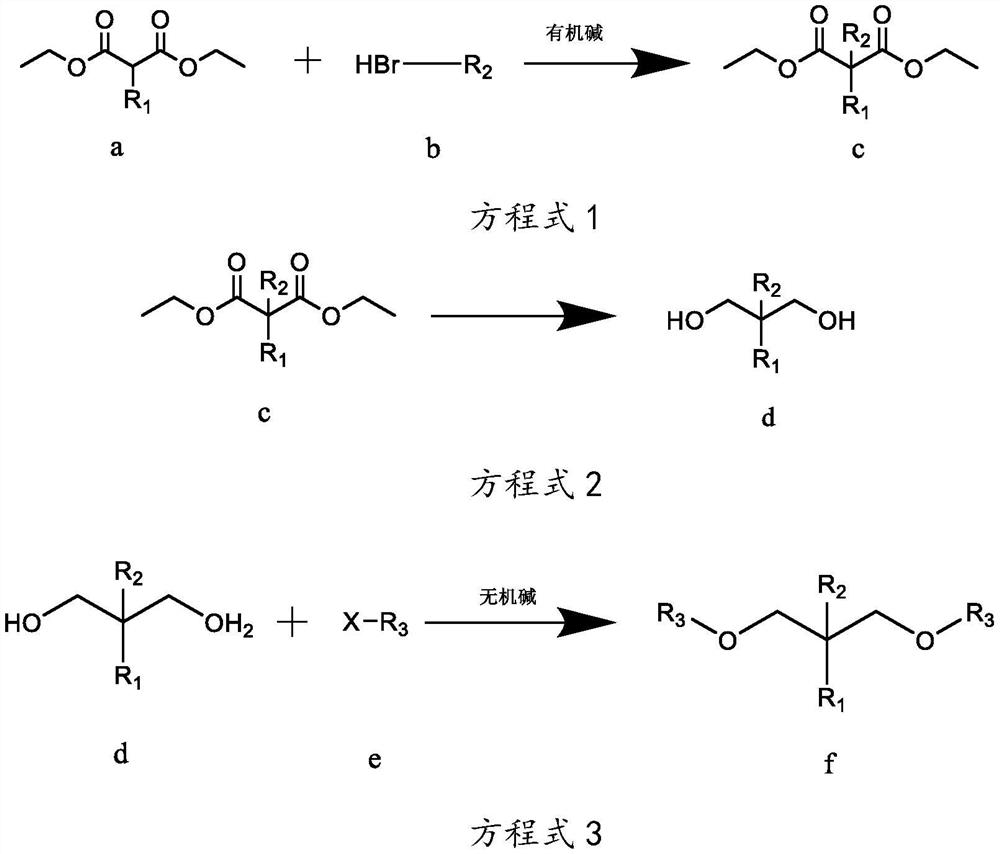
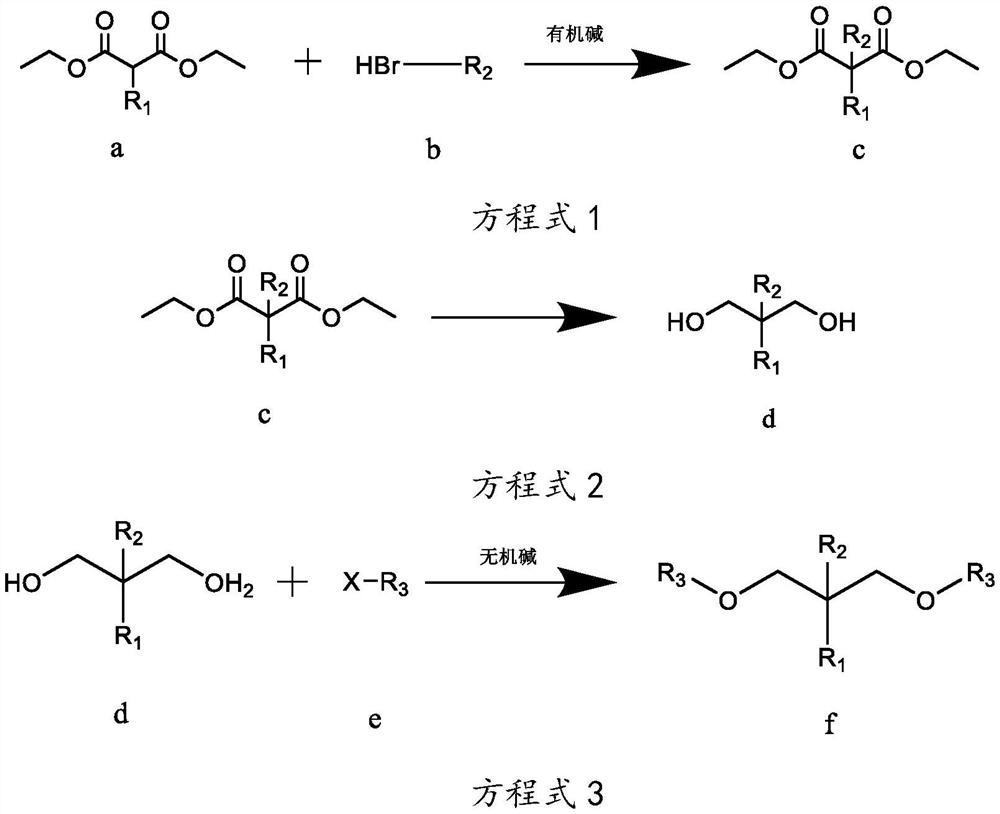
[0038] The preparation technology of isopropyl-2-isoamyl-1,3-dimethoxypropane, the reaction equation is as follows:
[0039]
[0040] Wherein, R1 is isopropyl, R2 is isopentyl, and R3 is methyl.
[0041] The concrete reaction steps of above-mentioned reaction equation are as follows:
[0042] Step A, as shown in equation 1, under the protection of inert gas at room temperature, weigh 260g of sodium ethoxide-ethanol solution with a mass concentration of 17%, add it to a 3L reaction flask, and add 101g of compound a isopropylmalonic acid dropwise diethyl ester in the reaction flask, keep the temperature at 10-35°C, after the dropwise addition, stir for 2-4h; gradually add 90.5g of compound b bromoisopentane dropwise into the reaction flask, and monitor the reaction by gas chromatography Progress, when the mass concentration of diethyl isopropylmalonate of compound a is measured to be less than 5%, stop dripping compound b bromoisopentane to obtain reaction mixture 1; filter ...
Embodiment 2
[0049] The preparation process of isopropyl-2-isobutyl-1,3-dimethoxypropane, the reaction equation of this embodiment is the same as the reaction equation of embodiment 1:
[0050] The difference from Example 1 is that R1 is isopropyl, R2 is isobutyl, and R3 is methyl.
[0051] The specific preparation steps of isopropyl-2-isobutyl-1,3-dimethoxypropane in this embodiment are as follows:
[0052] Step A, under the protection of an inert gas at room temperature, weigh 1532g of potassium tert-butoxide-tetrahydrofuran solution with a mass concentration of 13.05%, add it to a 3L reaction flask, and add 296g of compound a diethyl isobutylmalonate dropwise to In the reaction flask, keep the temperature at 10-35°C. After the dropwise addition, stir for 2-4h; add 201g of compound b bromoisopropane dropwise into the reaction flask, and monitor the progress of the reaction by gas chromatography analysis, and measure the compound a isopropane When the mass concentration of butyldiethylma...
Embodiment 3
[0059] The preparation process of 4,4-bis(methoxymethyl)-2,6-dimethylheptane, the reaction equation of this embodiment is the same as the reaction equation of embodiment 1:
[0060] The difference from Example 1 is that R1 is isobutyl, R2 is isobutyl, and R3 is methyl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com