Preparation and application of dopamine-coated urchin-shaped manganese dioxide hollow microspheres
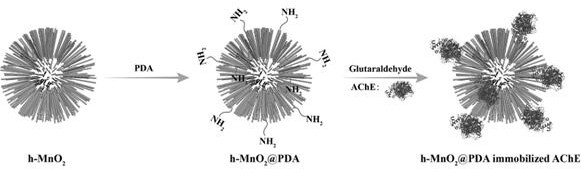
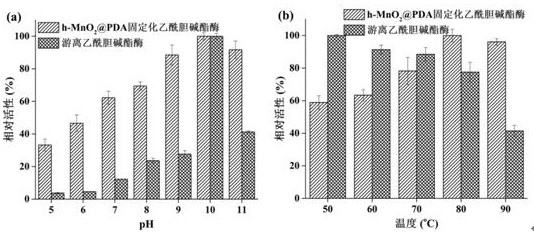
A technology of manganese dioxide and dopamine, which is applied to the application of the immobilized acetylcholinesterase in the screening of acetylcholinesterase inhibitors in traditional Chinese medicine, and the immobilized enzyme carrier is used in the field of preparing immobilized acetylcholinesterase, which can solve the problem of enzyme immobilization efficiency Low cost, poor stability of free enzyme, etc., to achieve the effect of less sample consumption, rich active sites, and short diffusion path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
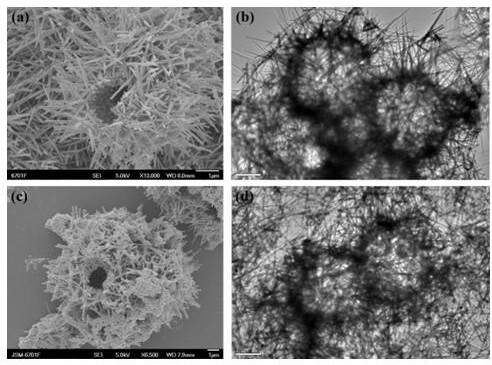
[0043] 1. Accurately weigh 0.4g of potassium permanganate, fully dissolve it in 20mL of ultrapure water; then add 2mL of concentrated hydrochloric acid drop by drop, and stir continuously at room temperature for 30 min; transfer the obtained mixed solution to polytetrafluoroethylene In an autoclave of ethylene, react at 120°C for 4 h; after the autoclave is cooled to room temperature, the product is collected by centrifugation, washed several times with ethanol and ultrapure water, and dried to obtain manganese dioxide hollow microspheres h-MnO 2 .
[0044] 2. Take 10mgh-MnO 2 Dissolve completely in 10 mL of Tris-HCl buffer solution (pH value 8.5), sonicate for 15 min; add 10 mg of dopamine hydrochloride, sonicate for 5 min, collect the product by centrifugation, wash several times with ethanol and ultrapure water, freeze-dry, Obtained dopamine-coated manganese dioxide hollow microspheres h-MnO 2 @PDA.
[0045] 3. Take 4mgh-MnO 2 @PDA, dispersed in 4mL phosphate buffer sol...
Embodiment 2
[0052] 1. Accurately weigh 0.4g potassium permanganate, fully dissolve it in 25mL ultrapure water; then add 4mL concentrated hydrochloric acid drop by drop, and stir continuously at room temperature for 40 min; transfer the obtained mixed solution to polytetrafluoroethylene In an autoclave of ethylene, react at 140°C for 3 h; after the autoclave is cooled to room temperature, the product is collected by centrifugation, washed several times with ethanol and ultrapure water, and dried to obtain manganese dioxide hollow microspheres h-MnO 2 ;
[0053] 2. Weigh 10mgh-MnO 2 , completely dissolved in 20 mL of Tris-HCl buffer solution (pH value 9.5), sonicated for 25 minutes; added 20 mg of dopamine hydrochloride, sonicated for 15 minutes, collected by centrifugation, washed several times with ethanol and ultrapure water, and dried. Obtained dopamine-coated manganese dioxide hollow microspheres h-MnO 2 @PDA;
[0054] 3. Weigh 4mgh-MnO 2 @PDA, dispersed in 8mL of phosphate buffer ...
Embodiment 3
[0057] 1. Accurately weigh 0.4g potassium permanganate, fully dissolve it in 30mL ultrapure water; then add 5mL concentrated hydrochloric acid drop by drop, and stir continuously at room temperature for 35 min; transfer the obtained mixed solution to polytetrafluoroethylene In a high-pressure reactor, the reaction was carried out at 130 °C for 4 h. After the autoclave was cooled to room temperature, the product was collected by centrifugation, washed several times with ethanol and ultrapure water, and dried to obtain manganese dioxide hollow microspheres h-MnO 2 ;
[0058] 2. Take 10mgh-MnO 2 , completely dissolved in 30 mL of Tris-HCl buffer (pH value 8), sonicated for 15 min; added 30 mg of dopamine hydrochloride, sonicated for 25 min, centrifuged to collect the product, washed several times with ethanol and ultrapure water, and dried. Obtained dopamine-coated manganese dioxide hollow microspheres h-MnO 2 @PDA;
[0059] 3. Take 4mgh-MnO 2 @PDA, dispersed in 10mL of phosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com