Neutralizing antibody against novel coronavirus, antigen-binding fragment thereof and application thereof
A technology of combining coronavirus and fragments, which is applied in antiviral agents, antiviral immunoglobulins, applications, etc., and can solve the problems of lack of specific and efficient antiviral drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Embodiment 1, the discovery of antibody
[0116] 1. Mouse immunization and preparation of immune antibody library
[0117] Female Balb / C mice aged 6-8 weeks were selected, and blood was collected from the tail vein of the mice to keep the background serum. The novel coronavirus S protein RBD domain recombinant protein (Beijing Yiqiao Shenzhou Technology Co., Ltd., product number: 40592-V08H5, referred to as recombinant RBD protein) was emulsified with Freund's complete adjuvant, and each mouse was injected intraperitoneally with 100 μg of recombinant RBD protein. Before the injection, the background serum was taken from the mice as a control. One week after the first immunization, the recombinant RBD protein was emulsified with Fisher's incomplete adjuvant, and each mouse was intraperitoneally injected with 100 μg of the recombinant RBD protein, and the blood was collected by docking the tail before the injection. After three rounds of immunization, shock immunization...
Embodiment 2
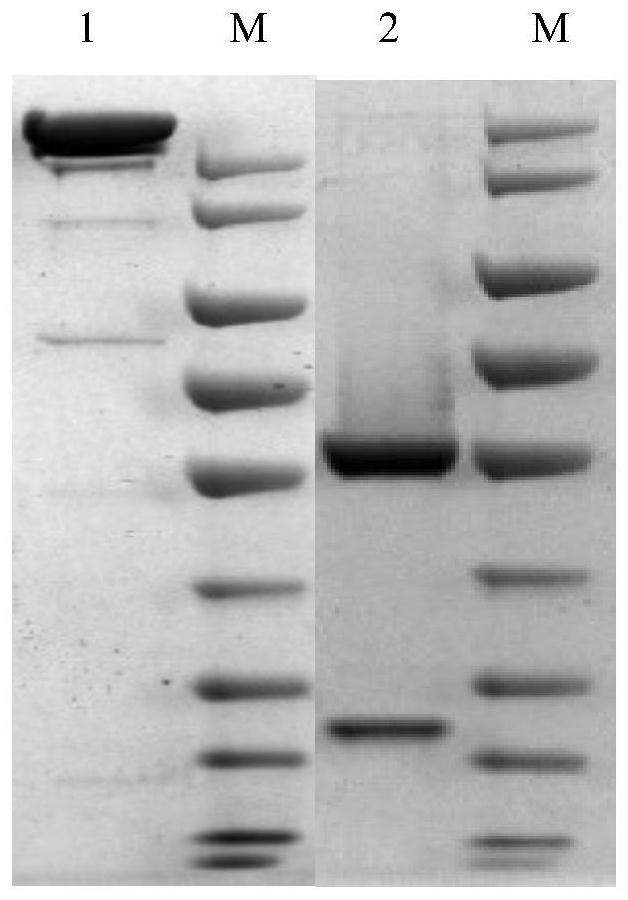
[0129] Embodiment 2, preparation of anti-new coronavirus chimeric antibody
[0130] A chimeric antibody is a chimeric antibody assembled from the variable region (V region) of a mouse antibody and the constant region (C region) of a human antibody. These two parts are relatively independent in terms of spatial structure, so their specificity and affinity are maintained. very good. In this example, a human-mouse chimeric whole antibody was prepared based on the murine single-chain antibody A-1F obtained in Example 1, named mhA-1F (the constant region of the heavy chain is IgG1, and the light chain is Kappa). The preparation process is as follows:
[0131] 1. Construction of recombinant plasmids
[0132] In order to express the chimeric antibody mhA-1F, the heavy chain expression vector and the light chain expression vector were prepared respectively:
[0133] 1. Combine the heavy chain variable region (VH) gene (1-354 of SEQ ID No.11) of murine single-chain antibody A-1F wit...
Embodiment 3
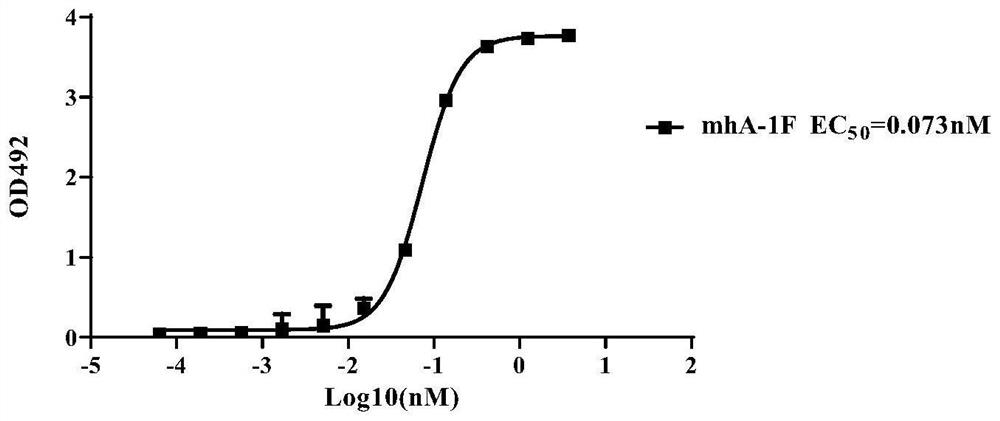
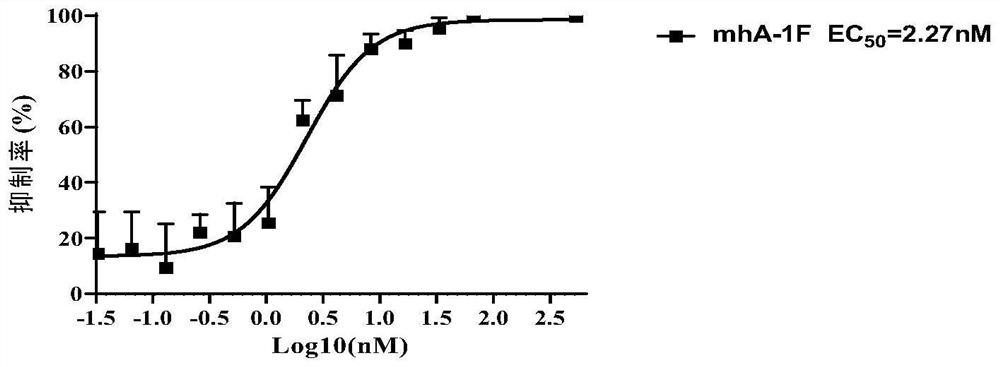
[0149] Example 3, Detection of specific binding ability of mhA-1F antibody
[0150] 1. Dilute SARS-CoV-2 RBD recombinant protein (commercialized RBD (Shenzhou, 40592-V08H5)) with carbonate coating buffer (pH 9.6) to 2ng / ul, add to the enzyme at a volume of 100 μL per well In the target plate (Corning, 9018), three replicate wells were set up for each experimental well, and coated overnight at 4°C.
[0151] 2. On the next day, wash the overnight coated ELISA plate with PBST 6 times, add PBS blocking solution containing 2% (mass percentage) skimmed milk powder, and incubate at 37°C for 2 hours.
[0152] 3. After the blocking is completed, discard the blocking solution, add 100 μL of 2-fold diluted mhA-1F antibody solution (initial concentration 1.2 nM) to each well, set up 11 gradients in total, incubate at 37°C for 90 minutes, and then wash the plate with PBST 6 times.
[0153] 4. After completing the above steps, take the ELISA plate, add 100 μL of 1:4000 dilution of HRP-lab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com