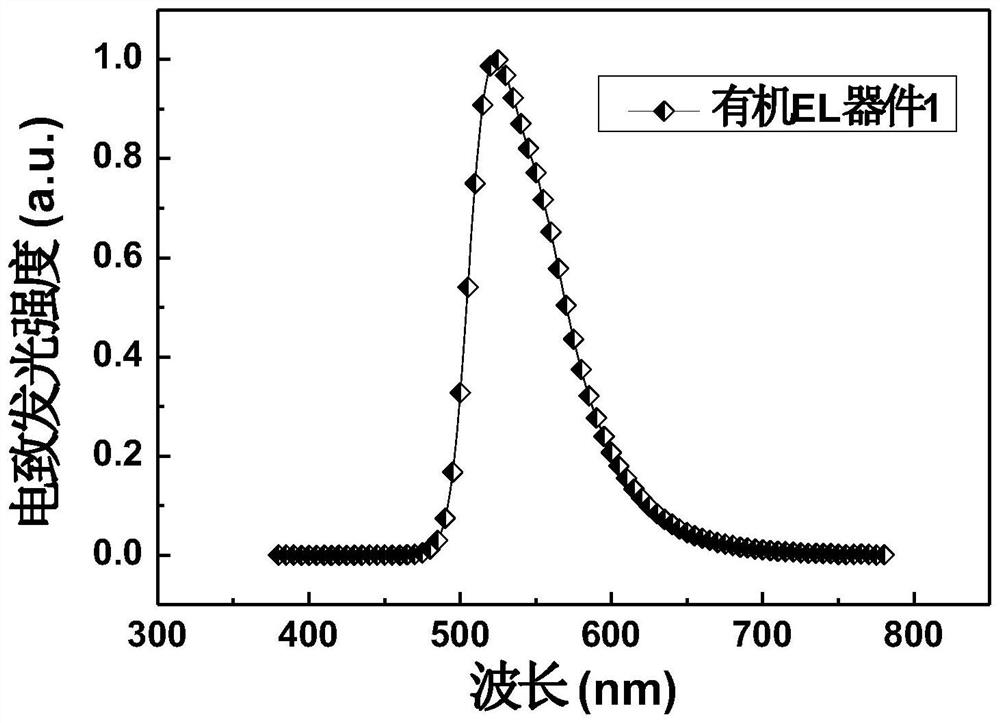
Organic electroluminescent material based on carbazole-9-yl-diphenylamine derivative and electronic device thereof
A technology of diphenylamine and derivatives, which is applied in the field of organic electroluminescent materials and electronic devices, can solve the problems of insufficient light-emitting layer composition, low luminous efficiency, and high driving voltage, and achieve low cost, simple preparation method, and high driving voltage. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Embodiment 1: the synthesis of compound 3
[0093] (Synthesis of Intermediate 1-1)
[0094] The synthetic route of intermediate 1-1 is as follows:
[0095]
[0096] Under nitrogen protection, potassium iodide (KI, 332 mg, 2 mmol), potassium periodate (KIO 4 , 6.9g, 30mmol), 4,4'-dibromodiphenylamine (3.3g, 10mmol), carbazole (1.7g, 10mmol) and 50mL of anhydrous acetonitrile (MeCN), and react overnight at room temperature. After the reaction was completed, the solvent was evaporated under reduced pressure, and the crude product was further purified by column chromatography (petroleum ether:dichloromethane=4:1 (V / V)). After distilling off the solvent, 3.4 g of white solid was obtained, with a yield of 70%. MS (EI): m / z: 491.87 [M + ]. Anal.calcd for C 24 h 16 Br 2 N 2 (%): C 58.56, H 3.28, N 5.69; found: C 58.54, H 3.30, N 5.66.
[0097] (Synthesis of compound 3)
[0098] The synthetic route of compound 3 is as follows:
[0099]
[0100] Under nitrogen p...
Embodiment 2
[0101] Embodiment 2: the synthesis of compound 14
[0102] (Synthesis of Intermediate 1-2)
[0103] The synthetic route of intermediate 1-2 is shown below:
[0104]
[0105] Under nitrogen protection, potassium iodide (KI, 332 mg, 2 mmol), potassium periodate (KIO 4 , 6.9g, 30mmol), diphenylamine (1.7g, 10mmol), 3,6-dibromocarbazole (3.3g, 10mmol) and 50mL of anhydrous acetonitrile (MeCN), and react overnight at room temperature. After the reaction was completed, the solvent was evaporated under reduced pressure, and the crude product was further purified by column chromatography (petroleum ether:dichloromethane=4:1 (V / V)). After distilling off the solvent, 3.6 g of white solid was obtained, with a yield of 74%. MS (EI): m / z: 491.76 [M + ]. Anal.calcd for C 24 h 16 Br 2 N 2 (%): C 58.56, H 3.28, N 5.69; found: C 58.53, H 3.32, N 5.67.
[0106] (Synthesis of Compound 14)
[0107] The synthetic route of compound 14 is as follows:
[0108]
[0109] Under nitroge...
Embodiment 3
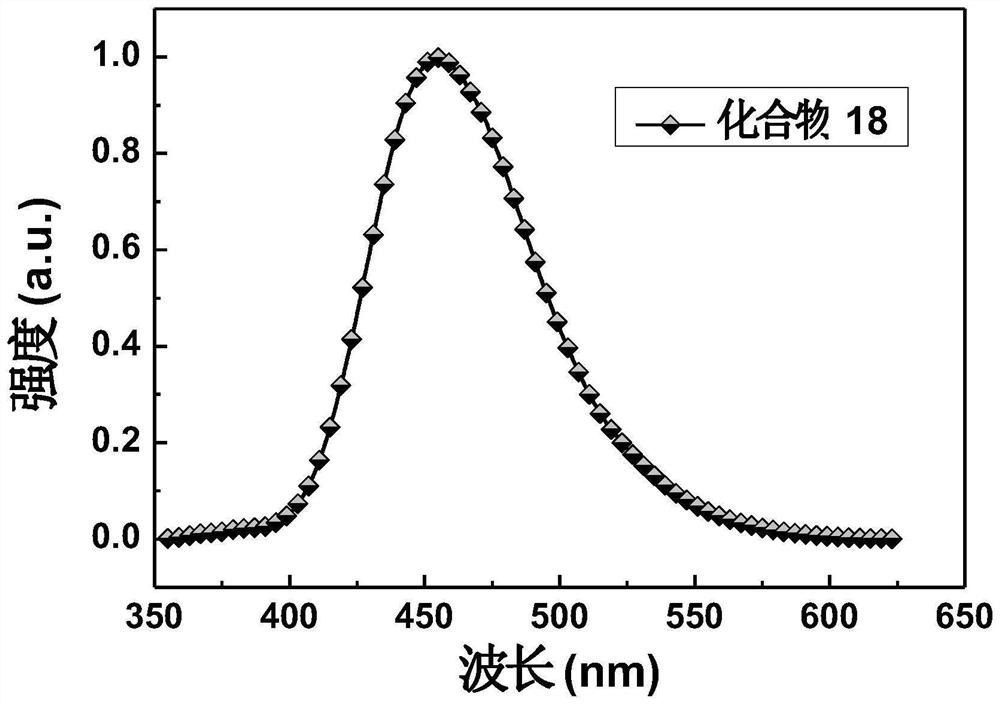
[0110] Embodiment 3: the synthesis of compound 18
[0111] (Synthesis of Intermediates 1-3)
[0112] The synthetic routes of intermediates 1-3 are shown below:
[0113]
[0114] Under nitrogen protection, potassium iodide (KI, 332 mg, 2 mmol), potassium periodate (KIO 4 , 6.9g, 30mmol), 4-bromodiphenylamine (2.5g, 10mmol), 3-bromocarbazole (2.5g, 10mmol) and 50mL of anhydrous acetonitrile (MeCN), and react overnight at room temperature. After the reaction was completed, the solvent was evaporated under reduced pressure, and the crude product was further purified by column chromatography (petroleum ether:dichloromethane=4:1 (V / V)). After distilling off the solvent, 3.9 g of a white solid was obtained, with a yield of 79%. MS(EI): m / z: 492.08[M + ]. Anal.calcd for C 24 h 16 Br 2 N 2 (%): C 58.56, H 3.28, N 5.69; found: C 58.55, H 3.29, N 5.66.
[0115] (Synthesis of Compound 18)
[0116] The synthetic route of compound 18 is as follows:
[0117]
[0118] Under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com