Naphthalene series liquid crystal monomer compound containing isothiocyano group as well as preparation method and application of naphthalene series liquid crystal monomer compound
A liquid crystal monomer and isothiocyanate technology, applied in chemical instruments and methods, liquid crystal materials, organic chemistry, etc., to achieve high birefringence, simple preparation method, and improve the effect of clearing point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
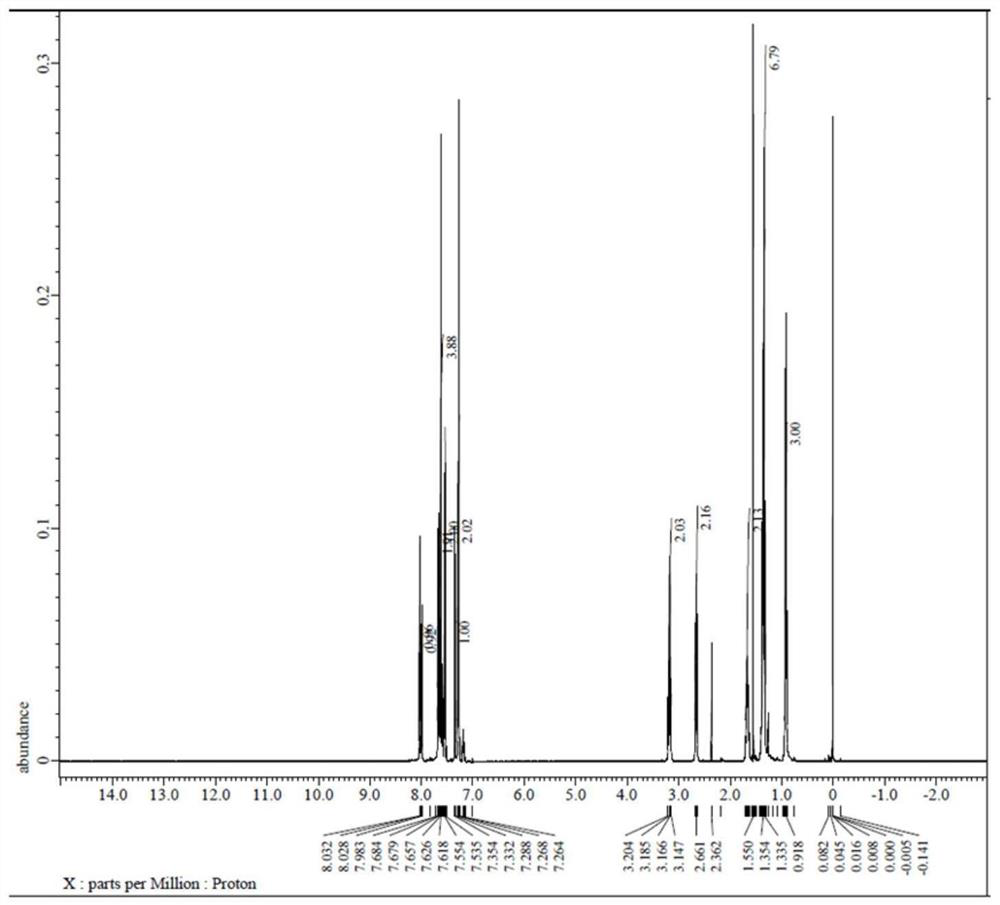
[0083] in formula middle
[0084] with R 1 The structure is -C 2 h 5 , R 2 The structure is -n-C 5 h 11 For example, Y 1 , Y 2 =H (abbreviated as 5BTNENCS), introduces the synthesis method of the isothiocyanate-containing naphthalene series liquid crystal monomer compound:
[0085] Step 1: Synthesis of intermediate m1:
[0086] Add 118.50g (0.5mol) of 2-bromo-6-methoxynaphthalene and 826g of dichloroethane into a 2L three-necked flask, cool down to -10-0°C, and add 79.98g (0.6mol) of aluminum trichloride. Add 47.10 g (0.6 mol) of acetyl chloride dropwise. After the addition is complete, the temperature of the system is raised to 0-10°C. Insulation reaction 2h. Hydrolyzed, the organic phase was washed to neutrality. The solvent was removed to obtain 122.0 g of light yellow solid m1, the GC purity was ≥94%, and the yield was 87.41%.
[0087] Step 2: Synthesis of intermediate m2
[0088] Add 122.00g (0.44mol) of intermediate m1 and 610.00g of diethylene glycol into...
Embodiment 2
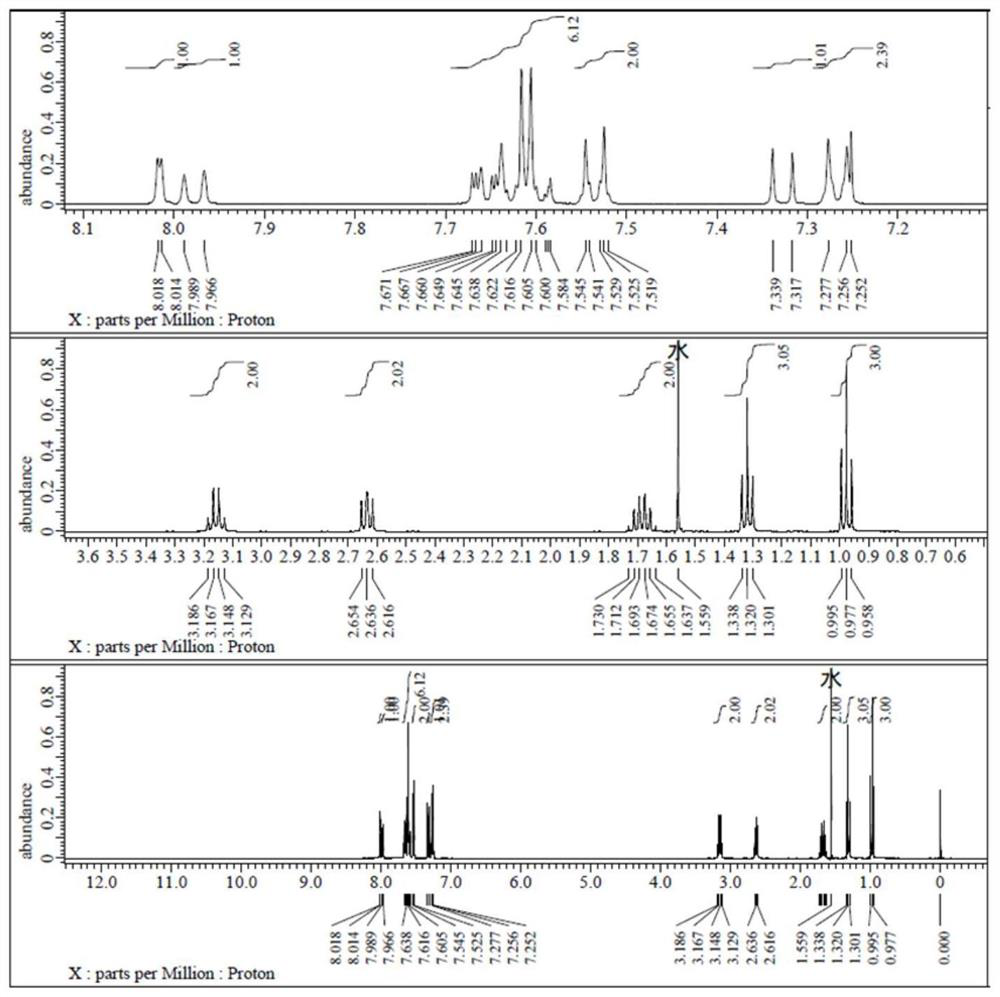
[0101] in formula middle
[0102] with R 1 The structure is -C 2 h 5 , R 2 The structure is -n-C 5 h 11 As an example (abbreviated as 5PTNENCS), the synthesis method of this isothiocyano-containing naphthalene series liquid crystal monomer compound is introduced:
[0103] Step 1: Synthesis of intermediate m1
[0104] Put 7.6g (43mmol) of 4-pentyl-1-phenylacetylene, 10g (39mmol) of the compound (intermediate m3 in Example 1), and 60.0g of triethylamine into a 250mL three-necked flask. Stir the system to dissolve, and use nitrogen to fully replace the air in the system. Catalyst 0.2804g (0.4mmol) PdCl 2 (PPh 3 ) 2 , 0.1524g (0.8mmol) CuI, 0.3144g (1.2mmol) PPh 3 , 0.4848g (4mmol) of DMAP was put into the system, the temperature was raised to an internal temperature of 85-90°C, and the reaction was kept for 8 hours. Triethylamine was distilled off under atmospheric pressure, 200 g of toluene was added, suction filtered, the filtrate was washed with water until neutr...
Embodiment 3
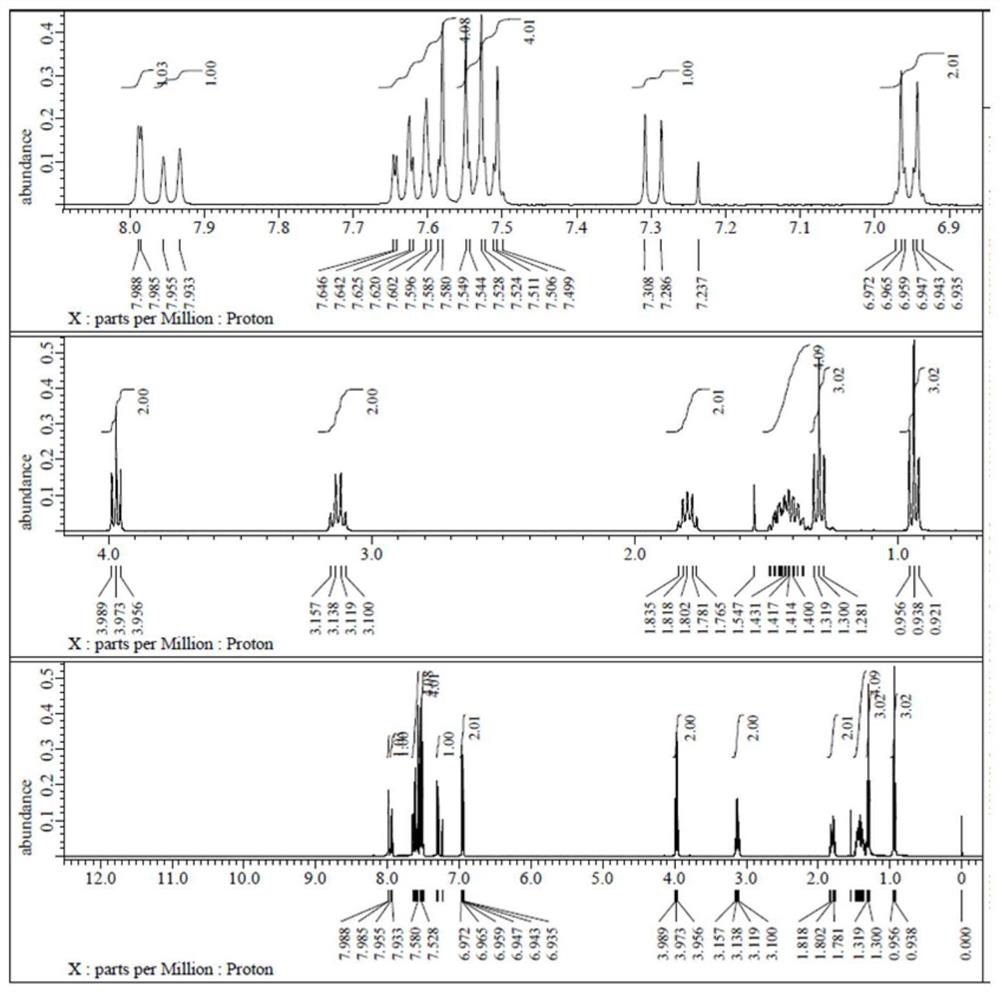
[0110] in formula middle
[0111] with R 1 The structure is -C 2 h 5 , R 2 The structure is -n-C 3 h 7 As an example (abbreviated as 3HPTNENCS), the synthesis method of this isothiocyano-containing naphthalene series liquid crystal monomer compound is introduced:
[0112] Step 1: Synthesis of intermediate m1
[0113] Put 8.8g (0.045mol) 1-ethynyl-4-(4-propylcyclohexyl)benzene, 7.5g (0.03mol) compound (intermediate m3 in Example 1), 60.0g triethylamine into 250mL three ports in the bottle. Stir the system to dissolve, and use nitrogen to fully replace the air in the system. Catalyst 0.2103g (0.3mmol) PdCl 2 (PPh 3 ) 2 , 0.1143g (0.6mmol) CuI, 0.2358g (0.9mmol) PPh 3 , 0.3836g (3mmol) of DMAP was put into the system, the temperature was raised to 85-90°C, and the reaction was kept for 8h. Triethylamine was distilled off under atmospheric pressure, 200 g of toluene was added, suction filtered, the filtrate was washed with water until neutral, and the organic phase ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Clear point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com