Placenta-derived endogenous polypeptide and application thereof
An endogenous, placental technology, applied in the field of biomedicine, to achieve the effect of promoting trophoblast cell invasion, promoting cell proliferation, and promoting placental trophoblast cell invasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Screening of endogenous polypeptides derived from human placenta
[0027] The placentas of severe preeclampsia patients (3 cases) and healthy pregnant women (3 cases) were collected at 30-39 weeks of gestation and delivered by cesarean section, and the maternal surface tissue pieces (about 1 cm in diameter) were collected and washed with sterile phosphate-buffered saline Blood is removed and the tissue is homogenized, ultrafiltered and desalted to obtain peptides. Peptides with significant differences between the two groups of placentas were screened (P value ≤ 0.05), and Blast2go software was used for GO bioinformatics analysis, through the KEGG website (KEGG; http: / / www.genome.jp / kegg ) to conduct pathway analysis on the polypeptide precursor protein, and use the ExPASy online tool (https: / / web.expasy.org / compute.pi / ) to calculate the isoelectric point (pI) and molecular weight (MW) of the polypeptide. Selecting two groups of placenta with high background level and ...
Embodiment 2
[0029] Entrust Shanghai Keyept Biotechnology Co., Ltd. to synthesize the polypeptide shown in SEQ ID NO.1 by solid phase method: ArgAsn Asp Glu Glu Leu Asn Lys Leu Leu Gly Gly Val Thr Ile Ala Gln Gly Gly ValLeu Pro Asn Ile (SEQ ID NO. 1)
[0030] This is a polypeptide consisting of 27 amino acids. The main biological parameters of the aforementioned polypeptide sequence obtained through online tools are as follows: the isoelectric point (PI) is 4.68, and the molecular mass (Mw) is 2819.21Da.
[0031] Take 10mg of polypeptide, dilute it with sterile double distilled water to 50mM-40°C and store it for later use.
Embodiment 3
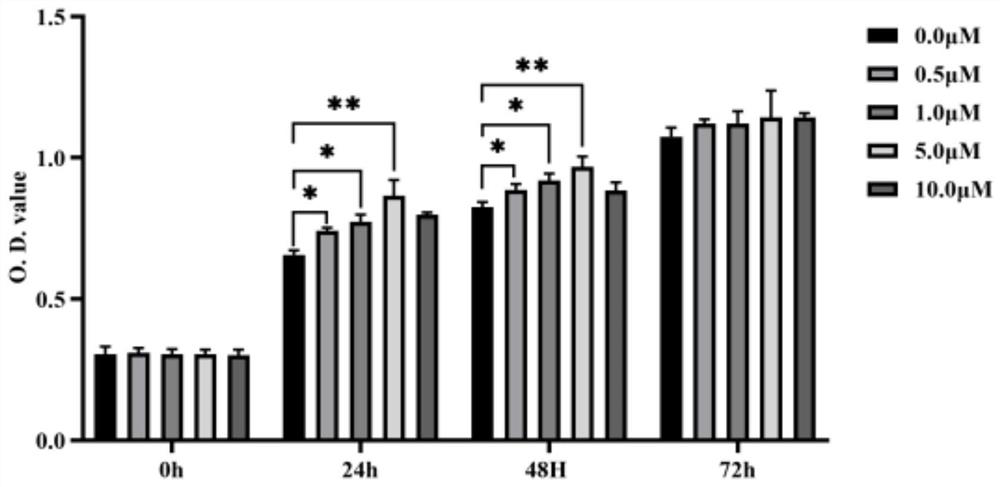
[0032] Example 3 CCK-8 method to detect the effect of polypeptide on the proliferation of human chorionic trophoblast cells (HTR-8 / Svneo)
[0033] In a 37°C, 5% CO2 incubator, human chorionic trophoblast cells were cultured, seeded in a 96-well culture plate at a density of 2000 cells / well, added 100 μl of medium / well, and treated with peptides (final concentration 0.5, 1.0, 5.0, 10.0 μM), the control group was treated with sterile double distilled water, and each group had 5 replicate wells, cultured in a 37°C incubator.
[0034] After 24 hours, 48 hours, and 72 hours, take out the 96-well plate, add 10 μl CCK-8 to each well to avoid the formation of air bubbles, and return to the 37-degree incubator to continue incubation for 2 hours. The absorbance was measured by a microplate reader, counted at a wavelength of 450nm, and the growth curve was drawn.
[0035] The result is as figure 1 As shown, it can be seen that the polypeptide can obviously promote the proliferation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com