Immunotherapy combination medicine for treating solid tumors
An immunotherapy, solid technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The embodiment of the present application provides the culture and passage of HFF (human foreskin fibroblast) and Pan02 pancreatic cancer tumor cells, specifically including:
[0049] 1. Resuscitate HFF and Pan02 cells, using complete medium (10% DMEM + 10% FBS + 1% penicillin / streptomycin solution) at 37°C, 5% CO 2 Cultivated in an incubator;
[0050] 2. When the confluence of HFF or Pan02 reaches 90%, add an appropriate amount of 0.25% trypsin-EDTA to cover the cell layer on the bottom of the culture flask, digest at 37°C for 4 minutes;
[0051] 3. After the cells are suspended and detached, add complete medium to stop trypsinization, then transfer the two types of cells to a new 15ml centrifuge tube, centrifuge at 1000rpm for 5min, and discard the supernatant;
[0052] 4. Then add complete medium to resuspend the cell pellet, dilute the cell suspension according to the ratio, and transfer it to cell culture dishes of different specifications such as cell culture fla...
Embodiment 2
[0054] The embodiment of the present application provides the subculture and preparation of non-replicating uracil auxotroph Toxoplasma gondii (NRTUA strain), specifically including:
[0055] 1. Resuscitate the non-replicating uracil auxotrophic Toxoplasma gondii (NRTUA avirulent strain) for use; when the confluence of HFF cells reaches more than 90%, replace the culture medium of HFF cells with a maintenance medium containing 250 μM uracil (mass fraction is 3%DMEM+mass fraction is 10%FBS+mass fraction is 1% penicillin / streptomycin solution), then 50×10 4 NRUTA strains were added to the HFF cell culture flask;
[0056] 2. Place the medium containing HFF cells inoculated with NRUTA at 37°C, 5% CO 2 Cultivated in an incubator, NRTUA is completely broken and passaged every 4 to 5 days;
[0057] 3. After breaking the NRUTA, use a 3 μm diameter filter to filter out cell debris, collect the filtered NRTUA tachyzoites, and resuspend them in PBS to adjust the concentration of NRTUA ...
Embodiment 3
[0059] The embodiment of the present application provides the subcutaneous tumor formation experiment of Pan02 tumor cell syngeneic mice, animal grouping and administration strategies of different drugs, including:
[0060] 1. Subcutaneous tumor formation experiment of Pan02 tumor cell syngeneic mice:
[0061] 1) Purchasing animals: purchase a sufficient number of 4-6 week-old C57BL6 / J female mice from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.;
[0062] 2) Animal breeding: SPF animal room A, North Campus of Sun Yat-sen University;
[0063] 3) Collect the Pan02 cells in the logarithmic growth phase, wash twice with PBS, digest with 0.25% trypsin-EDTA, resuspend the cells in PBS and count, adjust the viable cell density to 1×10 with PBS 7 cells / ml, use a 1ml syringe to inoculate 0.2ml of Pan02 cell suspension subcutaneously in the right flank of the abdomen of C57BL6 / J mice;
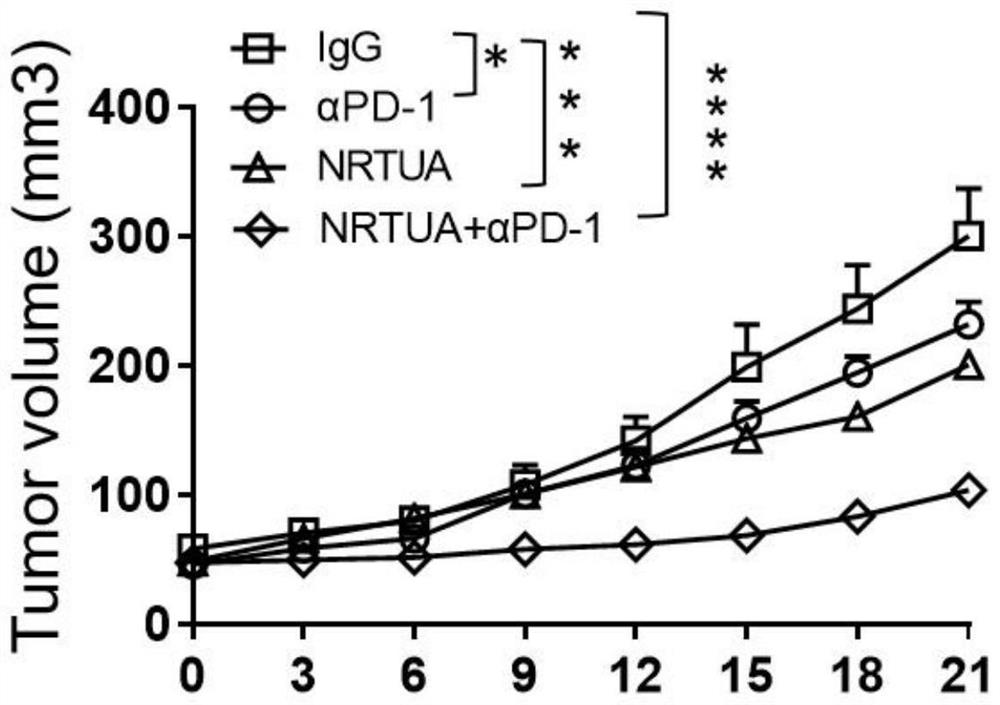
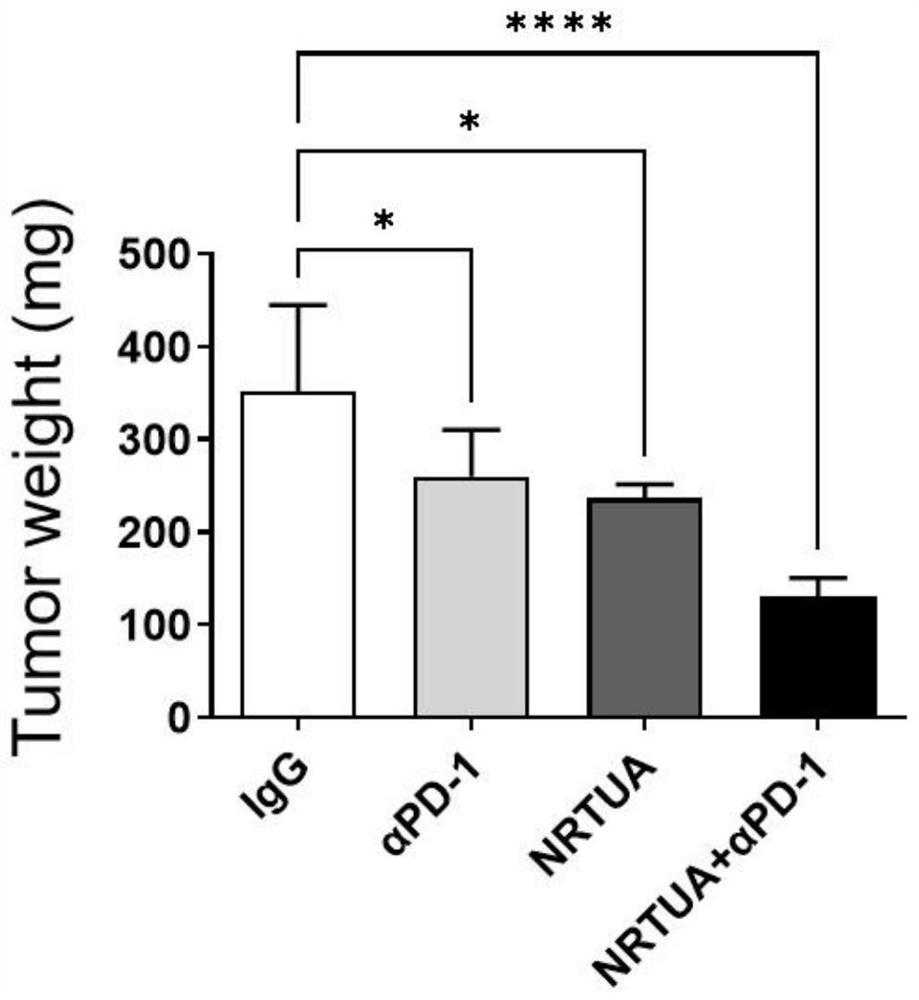
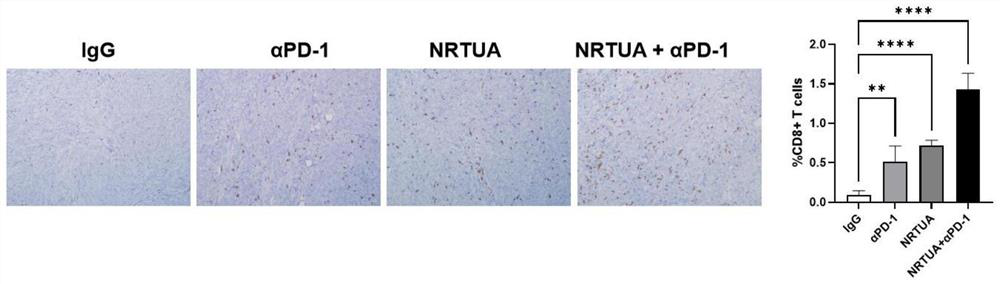
[0064] 4) Measure the volume of the mouse tumor with a vernier caliper every thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com