A kind of preparation method and application of human hematopoietic progenitor cells with B lineage differentiation potential
A technology of hematopoietic progenitor cells and differentiation potential, which is applied in the field of preparation of human hematopoietic progenitor cells, can solve the problems of undetectable antibodies, lack of B cells, and short time of B cells, and achieves the effect of improving induction efficiency, high application value and large quantity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1 Preparation of human pluripotent stem cells inducibly expressing exogenous RUNX1, HOXA9 and LHX2 genes
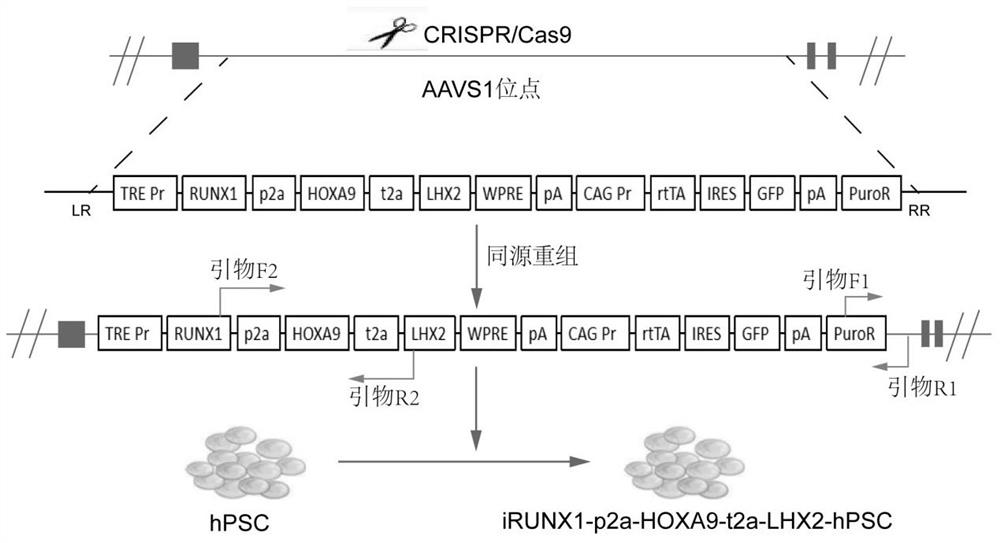
[0123] In this example, based on the CRISPR / Cas9 system, an inducible expression sequence was knocked into the AAVS1 site of human pluripotent stem cells (hPSC) by electroporation, and the knock-in sequence included RUNX1-p2a-HOXA9-t2a-LHX2 The tandem sequence, the fluorescent protein gene GFP and the puromycin resistance gene sequence for resistance screening, the schematic diagram is as follows Figure 1A shown.
[0124] The sgRNA and targeting vector were electroporated into hPSCs. After 20 h, hPSC medium containing puromycin (1 μM) was added, and the medium was changed every day. Wherein, the target sequence corresponding to the used sgRNA is shown in SEQ ID No.1.
[0125] SEQ ID No. 1: 5'-gtcaccaatcctgtccctagtgg-3' (hAAVS1-sgRNA).
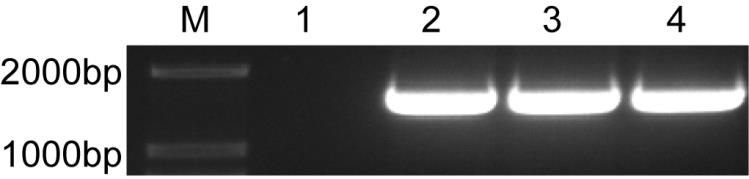
[0126] After 10 days of selection with puromycin, culture, expansion and passage were carried out in puromycin-free med...
Embodiment 2
[0134] Example 2 RUNX1, HOXA9 and LHX2 triple gene-modified human pluripotent stem cells induce differentiation to produce hematopoietic progenitor cells
[0135] The flow chart of the induction and differentiation of human pluripotent stem cells to generate hematopoietic progenitor cells (HPC) is as follows Figure 2A shown.
[0136] Using a monolayer culture-induced differentiation system based on stromal cell co-culture, the induced differentiation process is divided into three stages:
[0137] (1) The first stage is to induce human pluripotent stem cells to generate mesoderm cells (D0 to D2), Figure 2B Brightfield images of cells during induction of mesoderm cells are shown:
[0138] Culture in D0 medium and D1 medium respectively;
[0139] D0 medium was formulated as: 15 ng / mL hbFGF (Peprotech), 20 ng / mL rhActivin A (Peprotech), 30 ng / mL rhBMP4 (R&D), 4 μM CHIR99021 (Selleck) and 6 μM LY294002 (Selleck), balance It is TeSR-E6 basal medium (STEMCELL Technologies);
...
Embodiment 3
[0150] Example 3 Transplantation of iRUNX1-p2a-HOXA9-t2a-LHX2-hPSC-derived hematopoietic progenitor cells (B lineage seed cells) into immunodeficient mice and detection of B cell regeneration
[0151] Sort D16 to get CD34 + Cells were transplanted into immunodeficient NCG mice (Jicui Yaokang) with sublethal irradiation (2-3 Gy) by intravenous injection into the inner canthus at a dose of 1 million per mouse.
[0152] At 6, 8 and 10 weeks after transplantation, the peripheral blood of the recipient mice was collected by tail vein blood for flow analysis. The staining scheme was as follows: GFP, mTER119-PerCP / Cy5.5, hCD45-PE / Cy7, hCD19 - BV785, hCD33-PE, hCD3 / 4 / 8-APC and DAPI (all of the above antibodies were purchased from Biolegend). Data were analyzed using Flowjo software.
[0153] The result is as image 3 As shown in the figure, iRUNX1-p2a-HOXA9-t2a-LHX2-HPC can be used as B lineage seed cells to regenerate CD19 in recipient mice + B cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com